Checklist for Global De-Identification Compliance
Post Summary
De-identification is a critical process for protecting patient data while meeting global privacy regulations like HIPAA and GDPR. It involves removing or altering personal identifiers to prevent individuals from being identified. This guide provides a step-by-step checklist to help healthcare organizations navigate compliance, mitigate risks, and maintain trust when handling sensitive data across borders.
Key Points:
- Understand Regulations: Identify applicable laws (e.g., HIPAA, GDPR) and map data flows to ensure compliance.
- Apply Techniques: Use methods like Safe Harbor or Expert Determination to de-identify direct and quasi-identifiers.
- Governance: Assign roles, document processes, and establish policies for managing de-identified data.
- Monitor Compliance: Regularly reassess risks, track regulatory updates, and validate de-identification methods.
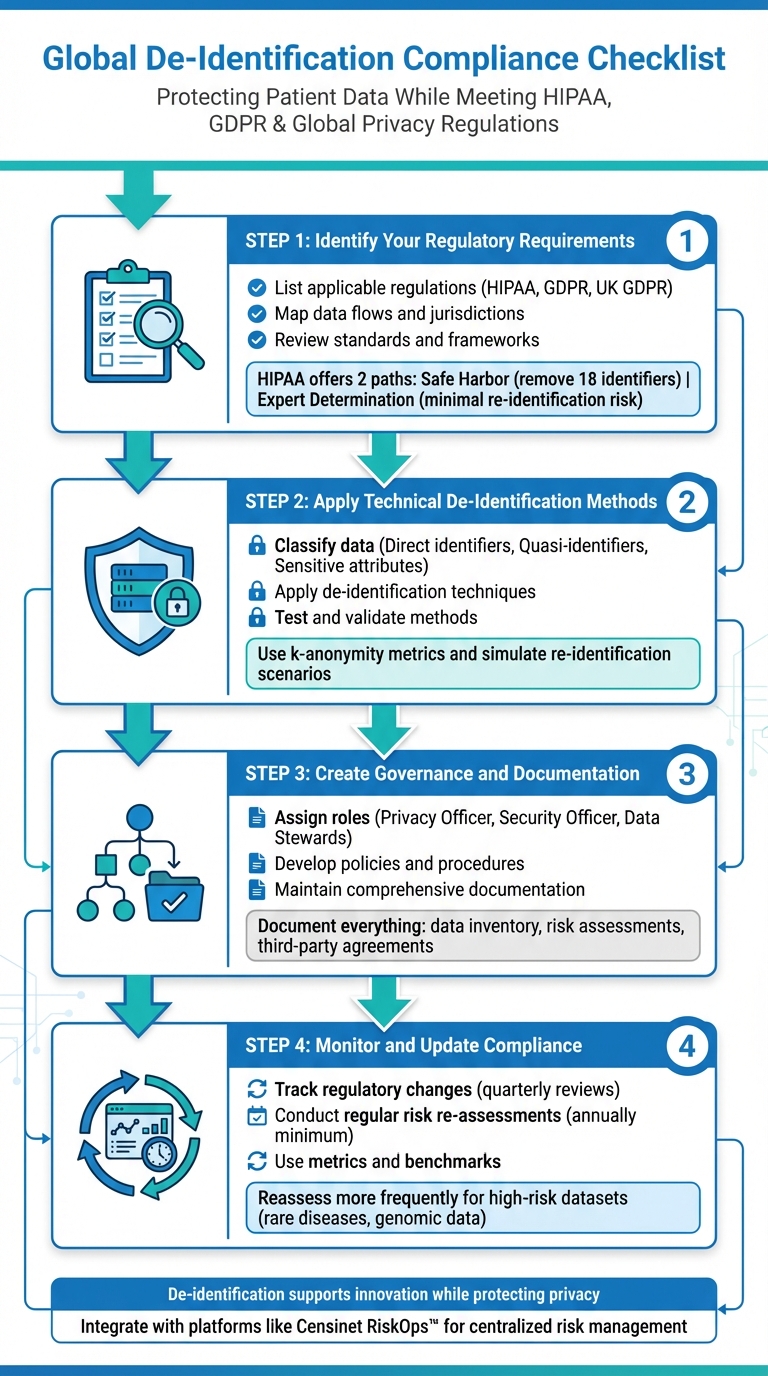
4-Step Global De-Identification Compliance Checklist for Healthcare Organizations
Step 1: Identify Your Regulatory Requirements
Start by pinpointing the laws and regulations that govern your data. This involves mapping where your data comes from, where it’s stored, and who accesses it. This foundational step ensures you can choose the right de-identification methods.
List Applicable Regulations
First, determine whether you fall under HIPAA as a covered entity or a business associate. If you do, HIPAA’s de-identification standards will apply to all protected health information (PHI). HIPAA offers two main paths for de-identification:
- Safe Harbor: Remove the 18 identifiers defined by HIPAA, such as names, detailed geographic information below the state level, most dates (except the year), and other personal details.
- Expert Determination: A qualified expert assesses and certifies that the risk of re-identification is minimal [3][6].
If you operate internationally, consider GDPR for data involving EU residents and UK GDPR for U.K. residents. These regulations differ from HIPAA. Under GDPR, truly anonymized data falls outside its scope, but pseudonymized data remains regulated [2][5]. To stay organized, create a regulatory matrix that matches each dataset with the jurisdictions and laws that apply.
Map Data Flows and Jurisdictions
Trace the journey of patient data from its collection points (like EHRs, apps, or devices) to where it’s stored (on-premises servers or cloud platforms) and processed (analytics tools or AI systems). Don’t forget to document how data is shared, whether with vendors or research collaborators [7].
For each data flow, note the physical locations (e.g., a server in Virginia or a cloud region in Ireland) and the legal jurisdictions involved (e.g., a vendor based in Canada or a research partner in Germany). This mapping helps you identify cross-border transfer requirements and situations where multiple regulations might overlap.
Review Standards and Frameworks
Ensure your de-identification practices align with established guidelines. For HIPAA, the HHS de-identification guidance outlines documentation standards for both Safe Harbor and Expert Determination approaches [3]. If you’re working under GDPR or UK GDPR, review their anonymization principles, which focus on context, the availability of auxiliary data, and the likelihood of re-identification [2][5].
For a broader perspective, the OECD Privacy Guidelines provide a framework that covers data collection, quality, purpose limitations, security measures, and accountability [5]. These guidelines encourage integrating de-identification into your overall data governance strategy. Make sure to document the legal basis for each dataset, the regulations that apply, and your chosen methods to maintain a clear audit trail [3][6].
Once you’ve clarified your regulatory requirements and aligned with the necessary standards, you’re ready to move forward with implementing technical de-identification techniques.
Step 2: Apply Technical De-Identification Methods
Once you’ve mapped out your regulatory requirements, it’s time to tackle the process of de-identifying your data. This involves organizing your data into categories, applying the right de-identification techniques, and ensuring your methods reduce the risk of re-identification effectively.
Classify and Inventory Data
Start by cataloging and classifying all the data elements you’re working with. Create a detailed data catalog that outlines each system (e.g., EHR, billing platform, research database) and its corresponding data fields. Then, sort these fields into three key categories:
- Direct identifiers: These are data points like names, Social Security numbers, medical record numbers, full addresses, phone numbers, and email addresses.
- Quasi-identifiers: These include data such as ZIP codes, dates of birth, gender, ethnicity, and diagnosis codes, which, when combined, could potentially pinpoint an individual.
- Sensitive attributes: This category covers information like diagnoses, medications, lab results, mental health notes, and genetic data that reveal health-related details.
To ensure nothing is overlooked, leverage automated data discovery tools, including scanners powered by natural language processing (NLP), to locate identifiers hidden in unstructured clinical notes or images. Document how data moves through your systems - from the source to integration layers and analytics tools - so that your classifications remain consistent throughout the pipeline.
Apply De-Identification Techniques
Once your data is classified, it’s time to apply specific de-identification methods tailored to each category:
- Direct identifiers: Suppress external direct identifiers entirely and replace internal ones with pseudonymous tokens using secure cryptographic techniques. Remove elements like names, phone numbers, email addresses, full street addresses, account numbers, and full-face images when sharing data externally. Store mapping tables in a separate, tightly controlled environment to maintain security.
- Quasi-identifiers: Generalize or aggregate these data points to lower the risk of linking records while still keeping the data useful for analysis. For instance, convert full birth dates into just the year or group them into age ranges (e.g., 0–4, 5–9). Combine ZIP codes into their first three digits, and for areas with fewer than 20,000 residents, replace the ZIP code with "000." Rare diagnosis or procedure codes can be grouped into broader "Other" categories to avoid creating uniquely identifiable records. Techniques like k-anonymity ensure that each combination of quasi-identifiers appears in multiple records, adding another layer of protection.
- Unstructured text: Use automated tools, including NLP-based methods, to identify and standardize protected health information (PHI) in unstructured text. Replace items like names, locations, IDs, and dates with standardized tokens (e.g., [NAME] or [DATE-YEAR]). Context filters can help avoid accidentally redacting clinical terms that resemble personal names (e.g., "Parkinson"). For medical images, remove or overwrite PHI in DICOM headers (e.g., patient name, ID, birth date, institution identifiers) and use pixel-level redaction to erase any annotations burned into the image itself.
Before moving forward, thoroughly test your de-identification methods to ensure they are working as intended.
Test and Validate De-Identification
Testing is critical to confirm that your de-identification approach effectively reduces the risk of re-identification. Use automated scans to identify and suppress table counts below five, which helps minimize risks. Metrics like uniqueness and k-anonymity can quantify how well your methods protect against potential linkages.
For additional confidence, simulate potential re-identification scenarios using external datasets like voter lists or public registries. This helps you evaluate whether an adversary could reasonably re-identify individuals. At the same time, test your key use cases to ensure that the data remains functional post-de-identification. Compare performance metrics before and after applying these transformations, and fine-tune your generalization levels to strike the right balance between privacy and usability.
Once validated, you’ll be ready to move on to governance and documentation practices in the next step.
Step 3: Create Governance and Documentation Processes
Building a solid governance framework is essential to ensure your de-identification efforts align with regulatory expectations. Agencies like HHS and privacy commissioners across different regions require healthcare organizations to follow a structured and well-documented process - not just tick off compliance checklists.
Assign Roles and Responsibilities
Start by assigning key roles to oversee and manage de-identification activities:
- A Privacy Officer should handle interpreting regulations, approving de-identification strategies, and managing risk assessments.
- A Security Officer can focus on technical safeguards, ensuring proper access controls, logging, monitoring, and addressing incidents involving de-identified data.
- Data Stewards or Data Owners should oversee major datasets. Their responsibilities include maintaining accurate data inventories, distinguishing between PHI and non-PHI, and ensuring that de-identification retains both clinical and analytical value.
For larger organizations, it may help to appoint a De-Identification Lead or Committee. This group, which could include statisticians or certified experts, can handle approving de-identification techniques, setting acceptable risk thresholds, and documenting compliance with HIPAA’s Expert Determination pathway, ensuring the re-identification risk is "very small."
Develop Policies and Procedures
Once roles are in place, formalize the operational procedures. Written policies should clearly outline:
- When de-identification is required, such as for secondary research, AI model training, or sharing data with external vendors.
- The approved methods for different scenarios, whether using HIPAA Safe Harbor, Expert Determination, or GDPR-compliant techniques like anonymization and pseudonymization.
- Minimum technical standards, such as removing the 18 HIPAA identifiers, applying date-shifting rules, or meeting k-anonymity thresholds.
These procedures should offer clear, step-by-step guidance for tasks like extracting data from source systems, applying approved de-identification tools, validating results, logging every execution, and securely storing the outputs. Each de-identification process should be tied to a specific purpose, retention timeline, and list of permitted recipients, all of which should be documented in a processing register.
Additionally, include steps for conducting initial re-identification risk assessments using recognized statistical methods. Document assumptions and any external data sources that could influence re-identification risks. Regular re-assessments are also crucial - whether annually or when datasets are updated or regulations change. Be sure to log findings, mitigation strategies, and expert evaluations for future regulatory reviews.
Maintain Documentation
Keeping accurate and up-to-date documentation is critical for compliance. This includes:
- A detailed data inventory that outlines all systems containing PHI, classifies data elements (e.g., direct identifiers, quasi-identifiers, or sensitive attributes), and tracks data flows across departments, facilities, jurisdictions, and external partners.
- Specification records for each de-identified dataset, which should include details about the source systems, identifier treatments, techniques applied, and parameter values. Pair these records with related risk assessments to show adherence to approved policies and expert standards.
When sharing data, agreements with third parties must clearly define how the data is classified and protected. These agreements should prohibit re-identification or re-linkage, specify permitted uses, outline geographic restrictions, set retention limits, and detail the security controls required for recipients. Platforms like Censinet RiskOps™ can streamline this process by centralizing assessments and maintaining a unified record of third-party risks for both de-identified and identifiable data.
sbb-itb-535baee
Step 4: Monitor and Update Compliance Practices
Once you've established governance and technical processes, the next step is to ensure your de-identification efforts stay effective over time. This isn't a "set it and forget it" situation - de-identification requires continuous monitoring. As regulations evolve, so must your methods to address new public datasets and changes in data sharing practices [3]. Here's how you can stay ahead by tracking regulatory changes, reassessing risks, and measuring the performance of your de-identification program.
Track Regulatory Changes
Designate team members - usually from legal, compliance, or privacy departments - to keep an eye on updates from key bodies like HHS, OCR, EU data protection authorities, and new U.S. legislation such as HIPRA [1][4]. For instance, HIPRA is expected to introduce unified national standards for de-identification that could exceed HIPAA requirements, including contractual clauses to prevent re-identification by third parties [1][4]. To stay compliant, hold quarterly meetings to translate these changes into actionable updates for your policies, technical protocols, and vendor agreements. Maintain a central tracker to log each regulatory update, its impact, and the steps taken in response. This tracker will also guide how often and how thoroughly you'll need to reassess risks.
Conduct Regular Risk Re-Assessments
Perform quantitative risk assessments, such as k-anonymity checks, small-cell analysis, or linkage simulations, at least once a year - or more frequently if your data conditions change [8]. For datasets with higher risks, like those involving rare diseases, genomic data, or small populations, reassessments should occur more often. In these cases, consider applying stricter methods, such as heavier generalization or suppression. For example, you might convert full dates to just month and year, suppress outliers, aggregate geographic details, or even replace sensitive data with synthetic alternatives [8]. Before implementing changes, test them to ensure they still support critical use cases, and document any trade-offs between privacy and functionality [8].
Use Metrics and Benchmarks
Use metrics to pinpoint areas for improvement. Track data like the number of datasets de-identified each quarter, average approval times, rework percentages, and any privacy incidents [8]. Tools like Censinet RiskOps™ can simplify this process by consolidating risk assessments across vendors and internal systems. These platforms also let you benchmark your organization's cybersecurity and privacy performance against industry standards, helping you monitor progress and address gaps.
Brian Sterud, CIO at Faith Regional Health, emphasizes: "Benchmarking against industry standards helps us advocate for the right resources and ensures we are leading where it matters" [9].
Conclusion
De-identification plays a critical role in protecting patient privacy while enabling the effective use of data. By following a structured checklist, organizations can align with global regulations like HIPAA, GDPR, Quebec Law 25, and emerging frameworks such as the European Health Data Space. This methodical process - understanding regulatory needs, applying technical solutions, establishing governance, and ongoing monitoring - helps minimize re-identification risks to acceptable legal levels. It also demonstrates accountability to regulators, partners, and patients [8].
When implemented properly, de-identification becomes a privacy control that supports innovation rather than hindering it. Techniques outlined in frameworks like HIPAA allow healthcare organizations to anonymize or transform sensitive data before sharing it for purposes such as AI model training or clinical research. This approach enables the use of large datasets without exposing personal information, while also mitigating risks associated with data breaches [8]. These safeguards contribute to a comprehensive, organization-wide risk management strategy.
To strengthen these efforts, integrate de-identification practices into your broader cybersecurity framework. Platforms like Censinet RiskOps™ can centralize risk assessments and streamline data-sharing workflows. Embedding de-identification into these workflows allows organizations to evaluate vendor security, include non-re-identification clauses in contracts, and continuously monitor risks related to PHI, supply chains, and digital health applications [8]. This structured approach not only ensures compliance but also fosters trust among patients and stakeholders across different jurisdictions.
To begin, incorporate this checklist into your privacy and security program. Assign clear responsibilities to compliance, legal, privacy, and security teams, and conduct a baseline review of your current de-identification practices against frameworks like HIPAA and GDPR. Start with a small set of high-priority use cases - such as de-identified data for AI development or multi-site research - to showcase the benefits while refining your processes [8].
FAQs
What is the difference between the Safe Harbor and Expert Determination methods for de-identifying data under HIPAA?
The Safe Harbor method ensures that data is de-identified by stripping away 18 specific identifiers, including names, addresses, and Social Security numbers. It provides straightforward guidelines and legal protection against HIPAA violations. However, it might lack the adaptability needed for handling more complex datasets.
On the other hand, the Expert Determination method relies on a qualified expert to assess the data and confirm that the risk of re-identification is minimal. This method is more adaptable and can be customized for specific scenarios, but it requires specialized knowledge and thorough documentation.
What are the key differences between GDPR and HIPAA when it comes to de-identification?
GDPR emphasizes data minimization and pseudonymization, meaning data can be re-identified if paired with separate, securely stored information. On the other hand, HIPAA mandates the absolute removal of identifiers, ensuring the data cannot be traced back to an individual under any condition.
Both frameworks share the goal of protecting sensitive information, but their methods differ. GDPR offers a more adaptable approach, allowing for controlled re-identification when needed. In contrast, HIPAA enforces stricter anonymity measures to prioritize patient data security.
What are the best practices for ensuring de-identification methods are effective?
When working to protect sensitive data through de-identification, it's essential to stick to some key practices. Begin by testing your methods on datasets you’re familiar with. This helps you gauge how well your approach balances privacy with maintaining the data's usefulness. Make it a habit to conduct re-identification risk analyses regularly. These analyses can reveal weak spots in your process, allowing you to address any issues before they become problems.
On top of that, establish continuous monitoring systems. These will help you stay aligned with global privacy standards and quickly adapt to new regulations or emerging risks. By following these practices, you can protect sensitive information while still keeping the data functional for its intended purpose.


