The CISO's Dilemma: How to Protect 1,000+ Connected Medical Devices with a Team of 5
Post Summary
Managing cybersecurity for over 1,000 connected medical devices with a small team is incredibly challenging. Hospitals rely on these devices for patient care, but most run on outdated systems, creating significant vulnerabilities. With the average healthcare data breach costing $10.93 million and impacting operations and patient safety, manual methods are no longer practical. Here's how small teams can handle this:
- Real-Time Inventory: Use automated tools to track every device, including its software and connectivity status, ensuring nothing is overlooked.
- Risk Prioritization: Focus on high-risk devices, like those critical to patient safety or handling sensitive data, using frameworks like IEC 62304.
- Automation: Leverage platforms like Censinet RiskOps™ to manage risks, monitor vulnerabilities, and streamline vendor assessments.
- Collaborative Workflows: Share responsibilities across IT, clinical engineering, and security teams to maximize efficiency.
- Key Metrics: Track inventory coverage, risk reduction, and response times to continuously improve security efforts.
Automation and a risk-based approach allow small teams to secure large device ecosystems effectively, protecting both patients and data while meeting regulatory requirements.

Medical Device Cybersecurity Statistics: Risks and Costs in Healthcare
Creating a Complete and Prioritized Device Inventory
Maintaining Real-Time Device Visibility
When managing the security of over 1,000 medical devices, the first step is understanding exactly what you’re protecting. A staggering 50% of healthcare organizations report poor asset and inventory visibility as a major weakness in their cybersecurity efforts [5]. Without real-time visibility, monitoring and protecting devices becomes nearly impossible [11].
Consider this: a single hospital room typically houses 15-20 connected medical devices, while larger healthcare systems may have as many as 85,000 medical and IoT devices spread across their networks [5]. This is where automated asset discovery tools come into play. These tools continually scan your network, identifying every connected device while capturing critical details like device model, software version, and connectivity status [3][4][5].
By eliminating human error, real-time monitoring ensures you have immediate access to device data during incidents [5]. To make this work seamlessly, your inventory system should pull data from various sources, such as IT asset management systems, clinical engineering databases, and network monitoring tools. It should also dynamically update to reflect new devices or those that have been retired [5].
Additionally, Software Bills of Materials (SBOMs) are now a regulatory requirement for medical device manufacturers. These provide an in-depth look at device components, software packages, and communication patterns [6][7]. Notably, 78% of healthcare organizations view SBOMs as essential or important for procurement decisions [6].
With a robust, real-time inventory in place, the next logical step is to assess and prioritize device risks.
Prioritizing Devices Based on Risk
Not all medical devices carry the same level of risk, and a small team cannot treat every device equally. A structured approach is essential to focus on the devices that matter most. Start by classifying devices based on their impact on patient safety. The IEC 62304 standard offers a helpful framework: Class A devices pose no injury risk if they fail, Class B devices could cause minor injuries, and Class C devices could lead to serious injuries or even death [2][10].
In addition to patient safety, consider factors like data sensitivity, known vulnerabilities, and how exposed the device is to the network [2][10]. This multi-layered evaluation helps pinpoint high-risk devices needing immediate attention. For instance, an internet-connected infusion pump with outdated software and access to patient data would rank high across all categories, making it a top priority for remediation.
Automating this risk assessment process is key to efficiently managing and prioritizing devices.
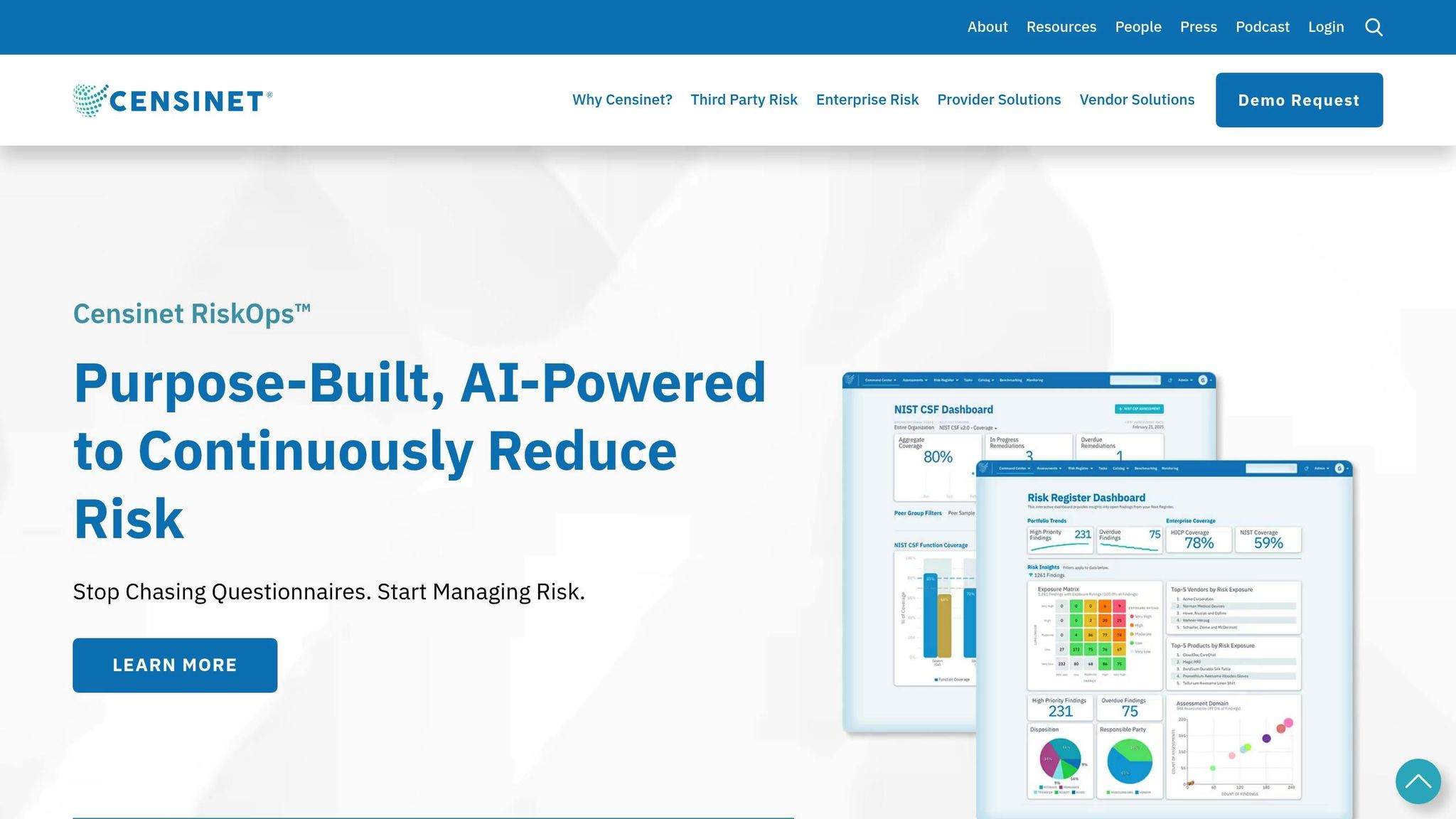
Using Censinet RiskOps™ for Device Management
Once you've prioritized risks, managing devices efficiently is the next challenge. Censinet RiskOps™ simplifies this by consolidating all device data into a single platform. Instead of juggling multiple tools and sources, your team can access real-time inventories, automated risk scores, and vulnerability tracking in one place. The platform continuously scans your network for new devices and applies risk scores based on patient safety, data sensitivity, vulnerabilities, and network exposure.
The addition of Censinet AI takes this a step further. It processes vast amounts of device data in real-time, identifying patterns and anomalies that could signal cyberthreats [8][10]. For a small team managing over 1,000 devices, this level of automation is a game-changer. It frees up your team to focus on high-risk devices and strategic decisions rather than getting bogged down by manual data entry.
Censinet's intuitive dashboards offer a clear overview of your entire device ecosystem, highlighting which devices need immediate attention and tracking progress on remediation efforts over time. This centralized approach ensures that your team can act swiftly and effectively to protect both patients and data.
Applying a Risk-Based Security Framework
Categorizing Devices by Risk Level
Once you've prioritized your inventory, the next step is to organize devices into risk tiers. This tiered approach ensures your team focuses its resources where they’re needed most. A helpful guideline is the IEC 62304 classification, which divides devices into three categories: Class A (no risk of injury), Class B (minor injuries possible), and Class C (serious injury or death) [10].
But don’t stop there. Consider additional factors like whether the device handles Protected Health Information (PHI), its exposure to networks, and known vulnerabilities. For example, an internet-connected infusion pump with outdated firmware and access to patient data would clearly fall into the high-risk category. On the other hand, a standalone blood pressure monitor that doesn’t store data or connect to a network would rank as low-risk. This risk assessment helps you build a matrix that guides your security strategy [13].
With nearly half of all medical devices in healthcare facilities connected to networks [12], categorizing by risk becomes a crucial step in protecting your organization.
Security Controls for Medical Devices
Each risk tier calls for tailored security measures, allowing your team to work more efficiently. For high-risk devices - like Class C equipment, PHI-handling devices, or internet-connected critical care tools - you'll need robust measures. These include network segmentation, multi-factor authentication, and continuous AI-driven monitoring [8][10][13]. These devices require real-time threat detection to quickly identify and respond to potential cyberattacks.
Medium-risk devices, while less critical, still need strong safeguards. Focus on access controls, regular vulnerability scans, and encrypted data transmission. However, these devices may not need the constant monitoring that high-risk devices demand [15].
For low-risk devices, stick to basic protections like enforcing password policies and scheduling periodic security updates.
Legacy devices that can’t be patched should be isolated using network segmentation to limit the damage of potential breaches [13]. Setting up a medical device lab can also help. In this controlled environment, you can perform detailed vulnerability scans that would be too risky to run on production devices [12]. Additionally, passive listening platforms can monitor network traffic and identify vulnerabilities without directly interacting with devices. This approach ensures your team focuses on the issues that matter most to your organization [12].
Implementing Controls with Censinet RiskOps™
Once your risk tiers are defined, automating the implementation of security controls becomes essential for a small team. This is where Censinet RiskOps™ steps in. The platform centralizes the process, mapping devices to the right controls, tracking progress, and continuously verifying that protections are in place [14].
With Censinet AI, your team gains real-time insights into network traffic and device behavior. The system flags anomalies that could signal compromised devices, ensuring your team focuses on genuine threats [8][10]. The platform’s dashboards provide a clear overview of which controls are active for each risk tier, which devices need attention, and where gaps remain in your security framework.
This level of automation transforms what could be an overwhelming task into a manageable workflow. Even with a small team, you can effectively secure more than 1,000 devices, operating as if you had a much larger staff.
Managing Vendor and Device Lifecycle Risks
Managing Vendor and Supply Chain Risks
The cybersecurity risks tied to connected medical devices don’t stop at the devices themselves. The larger ecosystem - encompassing vendors, manufacturers, software providers, and cloud services - requires just as much attention. Each player in this chain can introduce potential vulnerabilities. Medical devices often include components from multiple manufacturers, which makes updates and security management far more complex [7].
The numbers paint a concerning picture: 14% of connected medical devices operate on outdated or unsupported operating systems, and 21% rely on weak or default credentials [16]. On top of that, 53% of connected medical devices in hospitals have known vulnerabilities, with an average of 6.2 vulnerabilities per device [1]. If you’re managing a large inventory - say, over 1,000 devices - with a small team and no automation, pinpointing which vendor is responsible for specific vulnerabilities becomes nearly unmanageable.
Adding to the challenge, the FDA now mandates Software Bills of Materials (SBOMs) for all 510(k) and De Novo submissions as of October 1, 2023 [17]. These documents detail every software component in a device, offering greater transparency into what’s entering your network. While SBOMs are invaluable for identifying risks, manually collecting and analyzing them from numerous vendors can quickly overwhelm even the most dedicated teams.
Automating Vendor Assessments and Monitoring
When you’re juggling hundreds of suppliers, manual vendor assessments simply aren’t practical. That’s where tools like Censinet Connect™ and Censinet RiskOps™ come into play. These platforms automate the entire vendor assessment process, from initial risk scoring to ongoing monitoring. Vendors complete standardized security questionnaires directly within the platform, and their responses are automatically scored against your risk criteria.
The system doesn’t stop there - it tracks vendor compliance in real time. For example, it sends alerts when certifications expire or when new vulnerabilities are detected. This continuous monitoring ensures you’re not relying on outdated, once-a-year assessments. With 92% of healthcare organizations experiencing at least one cyberattack in 2024, and 69% reporting disruptions to patient care [16], staying ahead of vendor risks is critical. Automation not only keeps you informed but also lightens the workload across all risk management efforts.
Reducing Workload with Censinet AI
Censinet AI takes automation a step further, reducing tasks that once took days to just seconds. When vendors submit security documentation, the AI steps in to validate evidence, extract critical details from lengthy compliance reports, and pinpoint control gaps - even identifying risks from fourth-party vendors that traditional assessments might miss.
The system doesn’t just identify risks - it ensures the right people are informed. For instance, if a connected device is flagged with a critical vulnerability, the AI immediately notifies both your clinical engineering team and the device manufacturer. It also provides all relevant documentation, streamlining the response process. For a small team of five, this kind of automation not only cuts down on manual work but ensures swift action on emerging risks. Think of it as "air traffic control" for vendor management, with a centralized dashboard that gives you a clear, real-time view of all vendor risks in one place.
sbb-itb-535baee
Maximizing Efficiency for a 5-Person Security Team
Defining Roles and Workflows for Device Security
Securing all medical devices with just a five-person team is an enormous challenge. The solution? Collaboration. Establish a Medical Technology/IoT Management Committee that includes representatives from clinical engineering, IT, and security. This team approach spreads the responsibility for device security across departments, ensuring no single team is overwhelmed [18].
A well-defined workflow is key. Cover the entire lifecycle of each device. For instance, clinical engineering can flag new devices for review, security can evaluate vendor documentation and assess risks, and IT can handle network integration. By working together from the start, you ensure security considerations are baked into the process rather than added as an afterthought.
Measuring Performance with Key Metrics
Once roles are assigned, tracking performance becomes essential. Focus on two key metrics: device inventory coverage and risk reduction.
- Device inventory coverage measures how many medical devices on your network are accurately identified and cataloged [9][20]. In environments with over 1,000 devices, knowing exactly what’s connected at any moment is crucial.
- Risk reduction evaluates how quickly vulnerabilities are addressed and how your overall risk posture improves over time [9][20].
Given that attackers can lurk undetected for an average of 194 days [19], it’s also important to monitor your team’s responsiveness. Metrics like Mean Time To Detect (MTTD) and Mean Time To Respond (MTTR) will show whether your team is getting faster at identifying and neutralizing threats.
Using Censinet Dashboards for Oversight
To simplify oversight, centralized dashboards can make a huge difference. Censinet RiskOps™ consolidates all critical data - device risks, vendor assessments, and vulnerabilities - into one easy-to-use dashboard [21]. This "single pane of glass" provides a clear view of your medical devices, including inventory details, security status, vulnerability levels, traffic patterns, and even physical locations.
This setup allows your team to act quickly when issues arise. It also makes reporting to leadership easier, offering real-time data on inventory coverage, critical vulnerabilities, and risk reduction trends. By automating routine security tasks, the platform frees up your team to focus on bigger-picture strategies rather than getting bogged down in manual work [21].
Conclusion: Protecting Medical Devices with Limited Resources
Managing over 1,000 devices with just a five-person team is no small feat. Consider this: 96% of hospitals rely on outdated systems or software with known vulnerabilities, and one in five medical devices operates on unsupported operating systems [23]. Clearly, manual methods can't keep up with the scale and complexity of these challenges. To address this, a shift to automated solutions is essential.
The path forward hinges on three core strategies: real-time visibility, prioritized risk management, and automation. Start by creating a live, up-to-date inventory of every connected device. From there, focus your efforts by prioritizing risks based on their likelihood, potential impact, and the cost of mitigation [22]. Finally, use automation to handle repetitive tasks like vendor assessments, vulnerability tracking, and compliance reporting.
The stakes are enormous. The average healthcare data breach costs a staggering $9.77 million [20], and attackers often remain undetected for an average of 194 days [19]. Small teams need tools that consolidate device risks, vendor assessments, and vulnerabilities into a single dashboard, enabling faster detection and response. For example, platforms like Censinet RiskOps™ simplify these processes, allowing your team to focus on high-level security planning instead of being bogged down by manual tasks.
Alongside these strategies, additional measures like network segmentation for legacy devices, dynamic SBOMs from manufacturers, and thorough vendor evaluations are crucial [23]. Without an integrated, tech-driven approach to manage these elements, even the most skilled teams can become overwhelmed. The key lies in working smarter - leveraging technology to handle routine processes so your team can focus on critical decision-making.
Securing medical devices is about more than compliance - it's about protecting patient safety. The combination of real-time device visibility, risk-based prioritization, and intelligent automation enables small teams to achieve the efficiency of much larger operations. By embracing these strategies, healthcare organizations can safeguard their medical device ecosystems, meet regulatory requirements, and ensure the highest level of patient care.
FAQs
How can small teams secure thousands of connected medical devices with limited resources?
Small teams can secure a large number of connected medical devices by focusing on prioritizing risks and using resources wisely. Start by pinpointing the most critical devices and vulnerabilities that need immediate attention. Leverage automation tools to handle tasks like asset tracking, system monitoring, and patch updates - this can save both time and effort.
Network segmentation is another essential step, as it helps restrict access and contain potential threats. Strengthen security further by enforcing multi-factor authentication and implementing strong access controls for sensitive systems. Regularly updating device software and firmware is crucial, as is ensuring data encryption to protect patient information. Lastly, establish a well-defined and actionable incident response plan to tackle breaches swiftly and minimize damage.
By adopting these strategies, small teams can create a strong cybersecurity framework tailored specifically to healthcare needs.
How can automation and platforms like Censinet RiskOps™ improve the management of connected medical devices?
Managing connected medical devices becomes much easier with automation and platforms like Censinet RiskOps™. By automating routine risk assessments and offering real-time threat detection, these tools allow small healthcare teams to stay ahead of potential issues while effectively managing large inventories of devices.
Censinet RiskOps™ centralizes data and simplifies teamwork, enabling quicker decisions and smoother coordination across teams. This reduces the need for manual work and ensures that critical vulnerabilities are resolved promptly. For organizations with limited resources, this means device management becomes both more efficient and secure.
Why is it important to have real-time visibility into connected medical devices in healthcare?
Real-time visibility into connected medical devices plays a crucial role in strengthening cybersecurity within healthcare settings. It allows teams to keep a constant watch on device activity, spot vulnerabilities early, and address potential threats before they become serious issues. This kind of vigilance not only safeguards sensitive patient data but also ensures that devices remain operational, reducing any interruptions to patient care.
By leveraging real-time insights, healthcare organizations can focus on the most pressing risks, identify suspicious activity, and stay aligned with security standards. These measures are essential for protecting both patients and the medical devices they depend on.


