ISO 27001 for Medical Device Vendors: Key Benefits
Post Summary
ISO 27001 is a globally recognized standard for managing information security risks, offering medical device vendors a structured framework to safeguard sensitive data and ensure patient safety. It aligns with HIPAA and FDA cybersecurity guidance, addressing risks like ransomware, firmware tampering, and supply chain vulnerabilities.
Why it matters:
- Protects patient safety: Reduces risks from cyber threats that could harm patients.
- Simplifies compliance: Streamlines adherence to HIPAA, FDA, and other regulations.
- Prevents breaches: Encourages proactive measures to minimize incidents.
- Boosts trust: Certification is often required by healthcare providers and opens doors to new contracts.
- Strengthens supply chain security: Ensures third-party risks are managed effectively.
ISO 27001 helps vendors reduce cybersecurity incidents, protect PHI, and improve operational efficiency, all while building trust in the healthcare market.
How ISO 27001 Improves Medical Device Cybersecurity
Risk-Based Control Selection
ISO 27001 pushes medical device vendors to evaluate cybersecurity risks across all parts of their device ecosystem. This includes everything from embedded software and firmware to cloud dashboards, APIs, and hospital network integrations. Instead of applying generic protections, vendors must customize their security measures based on the likelihood of threats and their potential impact - especially when patient safety or sensitive health information is at stake.
Take this example: if a threat modeling exercise uncovers the risk of unauthorized firmware modifications, the appropriate response would involve implementing cryptographic firmware signing and strict authentication protocols. This kind of risk-based approach ensures that security measures are aligned with patient safety concerns. ISO 27001 also extends this methodology to cloud infrastructures and CI/CD pipelines, ensuring that environments like source code repositories and remote monitoring systems are assessed and secured just as thoroughly as the devices themselves.
Integration with ISO 13485 and FDA Guidance
ISO 27001 doesn’t operate in isolation - it complements existing quality frameworks, making compliance efforts more seamless. It aligns well with ISO 13485 in areas such as document control, management reviews, internal audits, and CAPA (Corrective and Preventive Actions). This means medical device vendors can integrate security risk assessments and vulnerability management into their existing quality management systems. For instance, security issues discovered during postmarket surveillance can be treated as quality nonconformities and addressed through CAPA processes.
The FDA’s latest cybersecurity guidance emphasizes that cybersecurity is a critical part of device safety. It highlights the importance of practices like threat modeling, secure development, maintaining a software bill of materials (SBOM), and robust postmarket monitoring. ISO 27001 provides a structured framework that aligns with these regulatory expectations by requiring formalized risk assessments, documented security requirements, and control implementation. These elements can be directly tied to threat models and security designs in FDA submissions. Postmarket, ISO 27001 also supports FDA expectations with its focus on incident management, vulnerability tracking, and coordinated disclosure processes, enabling timely responses to cybersecurity threats in marketed devices.
Impact on Patient Safety
Cyberattacks on medical devices pose serious risks to patient safety. For example, a compromised infusion pump could result in incorrect dosing, ransomware might delay critical imaging procedures, or tampered diagnostic data could lead to improper treatment decisions. ISO 27001 tackles these dangers by ensuring that patient safety drives the selection of security controls. During risk assessments, clinical impacts of security threats are carefully evaluated to prioritize measures that protect patients.
Key safeguards like logging, monitoring, and predefined incident response plans help detect and address threats quickly, minimizing disruptions to clinical operations. Additionally, contingency and continuity plans ensure that essential medical functions can continue during cyber incidents, reducing the risk of treatment delays or errors. Together, these measures not only protect against vulnerabilities but also help maintain uninterrupted care, directly supporting the broader benefits discussed in upcoming sections.
Benefits of ISO 27001 for Medical Device Vendors
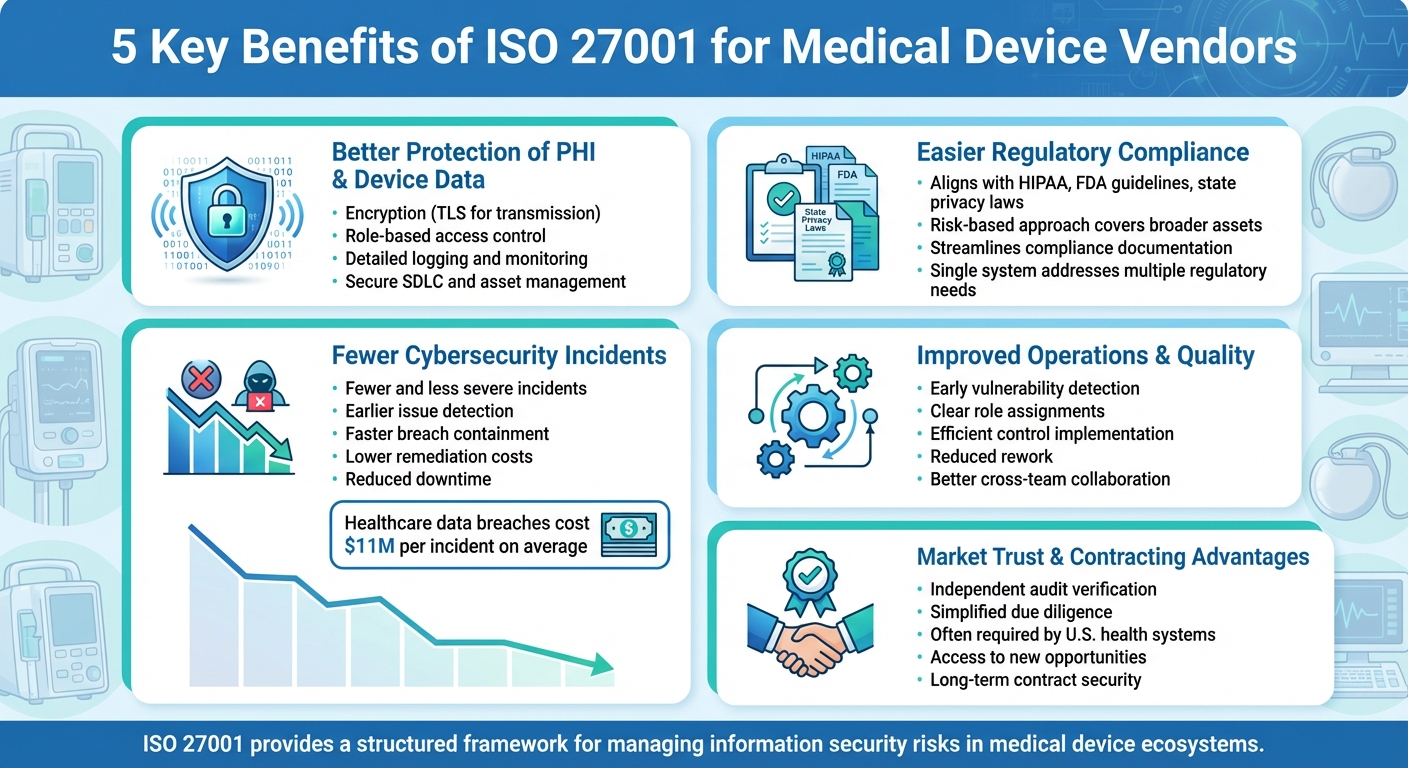
5 Key Benefits of ISO 27001 for Medical Device Vendors
Adopting ISO 27001 offers medical device vendors a range of advantages that help improve patient safety and operational efficiency.
Better Protection of PHI and Device Data
ISO 27001 mandates technical controls to safeguard patient health information (PHI) and proprietary device data. Key measures include encryption, role-based access control, and detailed logging [3][7]. Encryption ensures PHI and device telemetry are secure during storage and transmission (using TLS), minimizing the risks of data theft or device loss [3][2].
By enforcing least privilege and requiring strong authentication, the standard limits unauthorized access [3][2]. Additionally, logging and monitoring policies require collecting security logs from devices, gateways, and cloud systems. These logs are reviewed and analyzed to identify unusual activity or tampering [1][3]. Vendors align their Information Security Management Systems (ISMS) with product development, hosting, and support operations that handle PHI or critical device data. This ensures all access points are controlled, monitored, and technically enforced [1][4].
Easier Regulatory Compliance
ISO 27001 provides a structured framework that simplifies compliance with regulations like HIPAA, FDA guidelines, and state privacy laws. While HIPAA enforces specific safeguards for PHI, ISO 27001's risk-based approach covers a broader range of assets and creates repeatable processes [3][7]. Mapping ISO 27001 controls to HIPAA requirements helps vendors streamline compliance documentation and evidence.
Blue Goat Cyber highlights that adopting ISO 27001 demonstrates a commitment to patient safety and aligns with FDA expectations [1]. By integrating risk assessments, security controls, and documentation, vendors can directly connect their threat models and security designs to FDA submissions. This reduces redundancy, creating a single system that addresses multiple regulatory needs at once.
Fewer Cybersecurity Incidents
Organizations with ISO 27001-aligned ISMSs generally experience fewer and less severe cybersecurity incidents. This is due to continuous risk assessments, robust monitoring, and clearly defined response protocols [3][5][4]. These measures help prevent attacks like ransomware targeting connected medical devices and hospital systems [1][3]. Research shows that implementing ISO 27001 reduces breach events and associated financial losses by standardizing controls and response processes [2][3].
Life sciences providers also report that ISO 27001 helps detect issues earlier, contain breaches through network segmentation and access controls, and recover faster with tested disaster recovery plans [5][3]. Case studies reveal that certified ISMSs are linked to fewer breaches, reduced downtime, and lower remediation costs compared to less structured security programs [3][5][6]. Considering the high costs and reputational damage associated with healthcare data breaches, ISO 27001 provides a systematic way to mitigate these risks [3].
Improved Operations and Quality
ISO 27001 doesn't just enhance security - it also refines operational processes. It supports early vulnerability detection, clear role assignments, and efficient control implementation [1][4][5]. This reduces rework and fosters collaboration across engineering, quality, and security teams by establishing well-documented procedures.
IQVIA emphasizes that ISO 27001-certified providers can:
identify risk, assess their potential implications and put in controls to limit damage
This approach not only safeguards data but also strengthens corporate reputation [5]. Streamlined operations and well-defined processes contribute to smoother workflows and build trust in the market.
Market Trust and Contracting Advantages
ISO 27001 certification acts as a powerful signal of security maturity - something healthcare delivery organizations (HDOs) increasingly value during vendor evaluations and RFPs [5][4][6]. Certification demonstrates that a vendor has undergone independent audits, giving HDOs confidence in how PHI and device data are managed [5][2]. It also simplifies due diligence by addressing common security concerns upfront [5][4].
Large U.S. health systems and payers are now requiring ISO 27001 certification or its equivalent as a baseline standard. Certified vendors often gain access to new opportunities and secure long-term contracts that uncertified competitors might miss [4][6]. For example, Radar Healthcare attributes its expansion to the trust ISO 27001 certification fosters, noting it signals a:
genuine concern for data security
to customers [6]. Instant27001 further notes that ISO 27001 certification is increasingly becoming a standard business requirement in the healthcare and medtech industries [4]. This credibility strengthens vendor relationships with healthcare providers focused on patient safety and compliance.
| Benefit Area | How ISO 27001 Helps Medical Device Vendors |
|---|---|
| Better PHI & device data protection | Encryption, access control, logging, secure SDLC, asset management |
| Fewer cybersecurity incidents | Regular risk assessments, defined incident response, continuous monitoring |
| Market trust & contracting advantage | Recognized credential; often a prerequisite in healthcare vendor evaluations |
sbb-itb-535baee
How ISO 27001 Improves Supply Chain Security
ISO 27001 doesn't just strengthen internal cybersecurity - it also extends its reach to safeguard the supply chain. Medical device vendors often work with a complex web of suppliers, including contract manufacturers, software developers, cloud hosting providers, and maintenance partners. Each of these suppliers can introduce potential vulnerabilities, such as insecure firmware, misconfigured storage systems, or weak network defenses. By requiring vendors to inventory their assets, map data flows, and conduct thorough risk assessments for all external dependencies, ISO 27001 ensures that security measures extend beyond internal systems. This comprehensive approach helps protect sensitive data - like PHI, device telemetry, or proprietary information - at every touchpoint, ultimately supporting patient safety and regulatory compliance across the entire supply chain.
Addressing Supply Chain Vulnerabilities
The Annex A controls within ISO 27001 focus specifically on managing supplier relationships. Vendors are required to classify suppliers based on their risk levels, integrate security requirements into their contracts, and continuously monitor supplier performance. For medical device vendors, this means creating detailed risk registers for each supplier to evaluate threats such as compromised software updates, unvetted contract manufacturers with weak access controls, or cloud providers without healthcare-grade encryption. ISO 27001 also establishes technical standards tailored to each supplier's risk profile, including measures like multi-factor authentication, encryption (both in transit and at rest), vulnerability management, and activity logging.
The stakes are high - data breaches in the U.S. healthcare sector now cost an average of $11 million per incident, with many breaches linked to third-party vulnerabilities [3].
Third-Party Risk Management
ISO 27001 takes a lifecycle approach to managing third-party risks. Vendors are prompted to define clear policies for supplier management, perform risk-based due diligence during onboarding, and include security clauses in contracts. The framework also emphasizes ongoing monitoring of supplier performance. Due diligence commonly involves using standardized security questionnaires, reviewing certifications like ISO 27001 or SOC 2, and assessing how suppliers handle PHI and device-related data.
Throughout the relationship, vendors conduct periodic reviews, request audit reports or penetration test summaries, track remediation efforts, and update risk assessments as conditions evolve. At the end of a supplier relationship, ISO 27001 ensures secure data return or destruction and formal closure of risk records. These structured processes align with HIPAA and FDA expectations, providing a clear demonstration of due diligence in safeguarding PHI and ensuring device cybersecurity throughout the supply chain. Additionally, these processes set the stage for leveraging advanced digital tools to streamline supply chain risk management.
Using Risk Management Platforms
Managing a large number of suppliers with spreadsheets is no longer practical. Platforms like Censinet RiskOps™ can help implement ISO 27001-aligned supply chain governance by centralizing third-party risk assessments, automating evidence collection, and standardizing evaluations against control requirements. For medical device vendors, these platforms can categorize suppliers by risk - whether they process PHI, host cloud services, or develop device software - while simplifying reassessments and tracking remediation plans in a dynamic risk register. By digitizing questionnaires and securely storing supporting documentation, vendors maintain an auditable trail that supports both internal audits and external certification reviews.
James Case, VP & CISO at Baptist Health, highlighted the benefits of this approach:
Not only did we get rid of spreadsheets, but we have that larger community to partner and work with. [8]
This strategy allows healthcare organizations and vendors to benchmark supplier security, collaborate on reducing risks, and maintain continuous compliance with ISO 27001 standards. By strengthening supplier management, vendors not only enhance their reliability but also build greater trust in the marketplace.
Conclusion
Key Takeaways for Medical Device Vendors
ISO 27001 offers a structured, risk-based framework that not only secures medical device ecosystems but also prioritizes patient safety. By adhering to this standard, vendors can safeguard sensitive health and device data while showcasing a strong security stance during contract negotiations with healthcare organizations. Beyond meeting compliance requirements, ISO 27001 enhances operational efficiency by streamlining security processes, defining clear roles, and minimizing redundant efforts. Vendors with mature, standards-based security programs typically experience fewer and less severe incidents, leading to lower breach-related costs, reduced downtime, and less reputational damage. Additionally, certification provides a competitive advantage in RFPs, as more U.S. health systems are choosing ISO 27001–certified partners for their projects [4]. Incorporating the PDCA (Plan-Do-Check-Act) cycle alongside systems like ISO 13485 fosters resilience and supports long-term growth [9]. These advantages highlight the importance of maintaining a dynamic and proactive cybersecurity strategy.
Future Research and Industry Trends
Looking ahead, further research will help clarify ISO 27001’s evolving role in medical device cybersecurity. While current reports suggest that ISO 27001 reduces the frequency and severity of security incidents, more rigorous and long-term studies are needed to quantify its impact on device security and clinical outcomes [1]. Much of the existing evidence is either aggregated or qualitative, lacking the depth of controlled, multi-site trials. As medical devices increasingly rely on IoT and cloud platforms, the growing attack surface calls for continuous risk management practices. The industry is shifting away from basic compliance checklists toward a culture of constant improvement through regular audits, penetration testing, and risk assessments. Future studies should delve into device-specific security outcomes, patient safety metrics, and total cost of ownership to establish clearer benchmarks for assessing the long-term value of ISO 27001.
Matt Christensen, Sr. Director GRC at Intermountain Health, underscores the need for tailored solutions in healthcare security:
Healthcare is the most complex industry... You can't just take a tool and apply it to healthcare if it wasn't built specifically for healthcare [8].
FAQs
How does ISO 27001 improve the security of medical devices?
ISO 27001 plays a crucial role in bolstering the security of medical devices by offering a systematic framework for managing information security risks. It enables organizations to pinpoint vulnerabilities, enforce robust access controls, and establish measures to safeguard both sensitive data and the integrity of medical devices.
By emphasizing ongoing risk assessment and mitigation, ISO 27001 minimizes the likelihood of cyber threats while ensuring adherence to regulatory requirements. This approach not only prioritizes patient safety but also fosters confidence in the security of medical devices within healthcare environments.
How does ISO 27001 certification build trust for medical device vendors?
ISO 27001 certification allows medical device vendors to demonstrate their dedication to robust cybersecurity measures and alignment with international standards. This certification provides healthcare organizations, partners, and clients with confidence that both sensitive patient data and medical devices are safeguarded against potential threats.
By following the principles of ISO 27001, vendors highlight their proactive stance on protecting information. This not only helps build stronger relationships and reinforces trust but also boosts their reputation and creates opportunities for growth within the healthcare industry.
How does ISO 27001 help medical device vendors meet HIPAA and FDA requirements?
ISO 27001 offers a systematic framework for handling information security, making it easier for medical device vendors to meet critical regulations like HIPAA and FDA requirements. Its emphasis on safeguarding sensitive data and addressing risks directly aligns with HIPAA's Privacy and Security Rules, as well as the FDA's expectations for cybersecurity.
The standard encourages practices like conducting frequent risk assessments, developing strong incident response plans, and maintaining ongoing security enhancements. These measures help ensure that patient data and medical information remain confidential, accurate, and accessible when needed.


