Ultimate Guide to Post-Audit Remediation for IoT
Post Summary
Post-audit remediation for IoT involves addressing vulnerabilities and compliance gaps identified during an audit to enhance IoT security.
It ensures compliance, mitigates risks, and strengthens the security posture of IoT systems.
Key steps include identifying vulnerabilities, prioritizing risks, implementing fixes, and monitoring for ongoing compliance.
It reduces the risk of breaches, ensures regulatory compliance, and improves overall system resilience.
Tools like vulnerability scanners, compliance management platforms, and automated monitoring systems are essential.
Challenges include resource constraints, complex IoT ecosystems, and evolving regulatory requirements.
Post-audit remediation for IoT in healthcare is critical for addressing security risks and ensuring patient safety. This process involves fixing vulnerabilities identified during audits, such as weak authentication, outdated firmware, and insecure device configurations. Effective remediation protects patient care, secures sensitive data, and ensures compliance with regulations like HIPAA and HITECH.
Key Takeaways:
- What It Is: Post-audit remediation fixes IoT security gaps found in audits.
- Why It Matters: Protects patient safety, data security, and ensures regulatory compliance.
- Common Issues: Weak passwords, outdated software, poor network segmentation, and insufficient logging.
- Steps to Address:
- Identify and assess risks based on patient safety and data security.
- Create a prioritized plan considering clinical workflows and vendor timelines.
- Implement fixes with proper validation and documentation.
- Continuously monitor and track progress for future audits.
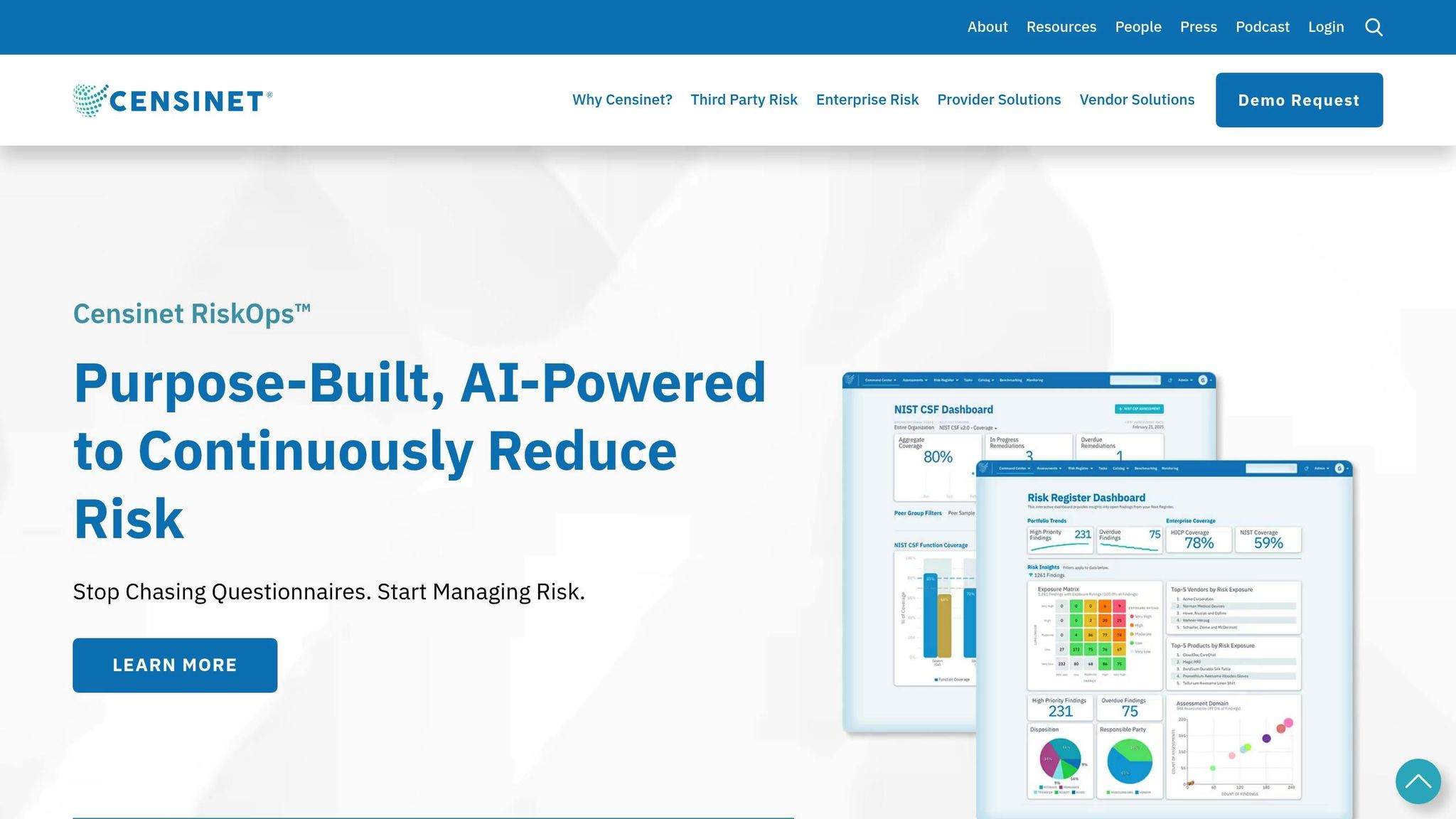
Tools like Censinet RiskOps™ simplify this process by automating risk assessments, managing vendor coordination, and tracking remediation progress in real time. This ensures faster resolutions and better compliance documentation, making IoT remediation more efficient and reliable.
IoMT - Internet of Medical Things - Healthcare IT Today Podcast Episode 106

Main Phases of the Post-Audit Remediation Process
Addressing healthcare IoT vulnerabilities after an audit requires a well-organized process that turns findings into actionable improvements. This approach ensures that security gaps are resolved while maintaining the critical balance between cybersecurity and uninterrupted patient care.
Vulnerability Identification and Risk Assessment
The first step toward effective remediation is a thorough analysis of the vulnerabilities uncovered during the audit. Each issue must be categorized based on its potential impact on operations.
For instance, vulnerabilities can be assessed in terms of patient safety, such as the risks posed by compromised infusion pumps, which could endanger lives. Data security is another key area, particularly when it comes to protecting patient health information (PHI) and ensuring compliance with HIPAA standards. This includes evaluating encryption protocols, access controls, and data retention practices. Lastly, consider operational impacts, like how a compromised patient monitoring system might lead to missed alerts or false alarms, potentially overwhelming clinical staff.
Risk scoring should weigh the likelihood of exploitation against the severity of potential outcomes. Devices with known active exploits should be prioritized, especially if they are connected to critical care units or handle sensitive data. The goal is to create a clear risk profile that guides the allocation of resources and establishes a timeline for addressing each issue.
Creating a Remediation Plan
Once risks are evaluated, the next step is to devise a detailed plan that translates these findings into actionable steps. This roadmap should take into account resource availability, clinical schedules, and vendor dependencies.
High-priority vulnerabilities should be addressed first, with IT, engineering, and clinical teams collaborating to ensure timely fixes. Lower-risk issues can be scheduled during routine maintenance or device updates. Budget considerations are essential, as emergency patches, device replacements, or additional security tools may be required.
Timelines should reflect the complexity of healthcare environments. For instance, critical vulnerabilities affecting patient safety might need resolution within 24-48 hours, while medium-risk issues could be addressed within 30-60 days. Lower-priority fixes can align with planned maintenance or equipment refresh cycles.
Vendor coordination is also crucial. Many IoT device manufacturers require formal patch requests, testing, or on-site support for updates. Establishing clear communication channels early on can prevent delays that might disrupt clinical workflows. Additionally, remediation activities should be carefully integrated into clinical operations to avoid interruptions in patient care. This might involve scheduling updates during low-activity periods or arranging backup equipment as needed.
Implementing and Validating Corrective Actions
This phase focuses on executing the remediation plan and validating its effectiveness. Coordination between technical teams and clinical staff is critical to ensure security improvements do not interfere with patient care.
Patches should be deployed in stages, starting with less critical devices to test their stability before rolling them out to life-critical systems. Vendor certifications, FDA approvals, and clinical testing should be completed before full implementation.
Network improvements, such as segmentation and micro-segmentation, can address many vulnerabilities. For example, creating dedicated VLANs for IoT devices or implementing zero-trust network access controls can limit potential attack vectors. However, these changes must be carefully tested to ensure they don't disrupt legitimate clinical communications.
Configuration hardening is another key step. This includes disabling unnecessary services, enforcing strong authentication protocols, securing communication channels, and setting up proper logging mechanisms. Each change should be tested to ensure it doesn’t compromise device functionality.
Validation is essential to confirm that the fixes have resolved the vulnerabilities without introducing new issues. This includes follow-up scans, penetration testing of updated systems, and verifying clinical workflows to ensure patient care remains unaffected.
Throughout this process, meticulous documentation is vital. Record all configuration changes, maintain logs of patch deployments, and update security policies to reflect the new procedures. This not only supports regulatory compliance but also provides a valuable reference for future audits.
Finally, continuous monitoring during and after implementation helps identify any unexpected issues. Real-time network monitoring, device performance tracking, and feedback from clinical staff ensure that the remediation efforts achieve their intended goals without compromising day-to-day operations.
Best Practices for Successful Remediation
Effective remediation is about more than just fixing vulnerabilities - it's about implementing strategies that protect operations while ensuring patient care remains uninterrupted.
Prioritizing High-Risk Issues
Start by focusing on vulnerabilities that pose the greatest risk, particularly those with high exploit potential and a significant impact on patient care. While tools like CVSS scores can help, healthcare settings often require additional context. For instance, a medium-severity issue in an infusion pump connected to an ICU network might need immediate action, whereas a similar vulnerability in a standalone fitness tracker could wait until routine maintenance.
Patient safety should always guide your priorities. Vulnerabilities affecting life-support devices, medication delivery systems, or critical monitoring equipment demand urgent attention. This could include unpatched firmware, weak authentication, or gaps in network segmentation that expose critical devices.
Assess the attack surface of each device. Devices with default passwords, unencrypted communication, or direct internet connections are particularly vulnerable. Their network position and access to sensitive data should dictate how quickly they are addressed.
Streamline resource allocation by grouping similar vulnerabilities across devices. For example, addressing weak encryption protocols across a fleet of patient monitors can provide widespread security improvements more efficiently than tackling each device individually. Partnering with vendors to ensure timely patch deployment further supports these efforts.
Working with IoT Device Vendors
Collaboration with IoT device vendors is crucial for successful remediation. Since manufacturers control firmware updates, security patches, and configuration changes, building strong vendor relationships is essential.
Establish clear communication channels with vendors and prepare standardized documentation to speed up patch approvals. In healthcare, patch management involves unique challenges, as medical devices often require additional validation and clinical testing to ensure updates don’t interfere with functionality. Close coordination with biomedical engineering teams and vendors is necessary to navigate these complexities. Understanding vendor timelines and certification requirements is key.
Service level agreements (SLAs) should reflect the critical nature of healthcare operations. Negotiate response times that align with your organization’s risk tolerance, especially for vulnerabilities that could impact patient safety. Some vendors offer expedited support for critical issues, but these arrangements often require prior planning and may involve extra costs.
Keep detailed records of all vendor interactions, including patch requests, response times, and outcomes. This documentation not only supports regulatory compliance but also helps identify vendors who consistently meet their security obligations, which can inform future partnerships and negotiations.
Coordinated vendor efforts contribute to a more comprehensive risk management strategy.
Connecting Remediation to Broader Risk Management
Effective remediation should seamlessly integrate into your organization’s overall risk management strategy. Treating remediation as part of a larger framework - not as a standalone activity - ensures that fixes align with organizational goals and meet regulatory demands.
Compliance mapping is a critical step in this process. Align remediation efforts with frameworks like the HIPAA Security Rule, the HITECH Act, and the NIST Cybersecurity Framework. This helps translate legal requirements into actionable technical controls while identifying gaps between your current security posture and regulatory expectations.
Incorporate IoT threat analysis into regional compliance strategies. Organizations that align risk modeling with regulatory requirements have seen a 30% improvement in mitigation response times compared to those relying on traditional assessments[1].
Regular gap assessments and readiness audits are essential for staying ahead of regulatory changes and identifying new vulnerabilities. These evaluations should cover not only technical controls but also related policies, procedures, and training programs that support IoT security.
When prioritizing risks, consider both technical vulnerabilities and compliance requirements. For example, a vulnerability that threatens patient health information confidentiality might take precedence due to HIPAA mandates, even if its technical risk score is moderate. This dual focus allows organizations to allocate resources effectively while meeting strict data protection laws.
Shared accountability is a persistent challenge in healthcare IoT environments. Security responsibilities often span multiple stakeholders, including healthcare providers, device manufacturers, IT teams, and third-party vendors. Clearly defining roles and responsibilities ensures that no aspect of the security ecosystem is overlooked.
Integrating remediation into governance, risk, and compliance (GRC) frameworks provides structure for continuous improvement. This includes setting metrics to measure the success of remediation efforts, creating reporting mechanisms for decision-makers, and using lessons learned from audits to strengthen future security practices.
sbb-itb-535baee
Using Censinet for Streamlined IoT Remediation
When it comes to addressing the challenges of post-audit remediation, Censinet RiskOps™ steps in as a game-changer. By combining automation with strategic oversight, this platform eliminates the inefficiencies of fragmented workflows. Instead of juggling spreadsheets and emails, healthcare teams can manage the entire remediation lifecycle - from identifying vulnerabilities to validating fixes - all within one cohesive system.
Key Features of Censinet RiskOps™ for IoT Remediation
Censinet RiskOps™ brings a range of features designed to simplify and streamline IoT remediation:
- Automated Risk Assessment and Workflow Orchestration: The platform evaluates vulnerabilities across IoT devices, applying healthcare-specific criteria that consider both patient safety and security risks. Tasks are automatically routed to the right stakeholders, such as biomedical engineers, while vendors are notified and progress is tracked through predefined workflows.
- Centralized Vendor Management: Managing IoT device manufacturers becomes easier with detailed vendor profiles, service level agreement tracking, and automated workflows for patch requests. Teams can batch update requests for devices from the same vendor and monitor response times - all from a single interface.
- Real-Time Progress Tracking: Dashboards provide instant visibility into remediation efforts, showing which vulnerabilities are being addressed, which require vendor action, and which have been resolved.
- Compliance Mapping: The platform aligns remediation activities with regulations like HIPAA and HITECH, automatically generating documentation for regulatory reporting as vulnerabilities are addressed.
Why Censinet Makes a Difference in Post-Audit Remediation
Censinet RiskOps™ delivers measurable improvements in efficiency, compliance, and risk management:
- Faster Remediation Timelines: Automating tasks such as risk assessment, stakeholder notifications, and progress tracking significantly reduces the time it takes to implement corrective actions after an audit.
- Improved Visibility Across Teams: Whether it’s IT security or clinical engineering, all stakeholders can access real-time updates on device status, vendor communications, and remediation progress. This shared visibility eliminates redundant efforts and ensures no detail is overlooked.
- Automatic Compliance Documentation: As remediation progresses, the platform generates audit trails to show due diligence, making regulatory inspections much smoother.
- Strategic Resource Allocation: With a clear view of IoT risks, teams can spot patterns, evaluate remediation strategies, and make informed decisions about where to focus security efforts.
By consolidating vulnerability scanning, vendor management, compliance tracking, and remediation workflows, Censinet RiskOps™ simplifies training and data management, creating a unified approach to IoT remediation.
How Censinet AITM Enhances Risk Mitigation
Censinet AITM introduces AI-powered tools to speed up risk assessments and remediation while keeping human oversight at the forefront. Here’s how it works:
- Quick Security Assessments: Vendors can complete security questionnaires in seconds, slashing the time needed to evaluate new or existing IoT devices after vulnerabilities are found.
- Automated Evidence Validation: Security documentation from vendors - like certificates or patch notes - is analyzed automatically. This feature saves time by extracting key information, allowing teams to confirm that fixes address vulnerabilities without manually sifting through lengthy reports.
- Actionable Risk Summaries: The platform generates detailed risk reports that highlight critical issues, suggest remediation actions, and prioritize steps based on patient safety concerns.
- Human-Guided Automation: AI recommendations are configurable, so teams can decide which actions require manual approval and which can be automated. This ensures that automation supports, rather than replaces, human decision-making.
- Advanced Workflow Orchestration: Critical findings are routed to the right stakeholders, ensuring that issues are addressed efficiently. When vulnerabilities span multiple devices or departments, the system coordinates parallel remediation efforts while maintaining centralized oversight.
- Real-Time AI Risk Dashboard: This dashboard consolidates remediation data into an easy-to-read interface, enabling quick decision-making and helping organizations refine their processes over time.
Tracking, Reporting, and Validating Remediation Results
After implementing and validating security measures, the process isn’t complete without thorough tracking and documentation of remediation efforts. This step is critical for maintaining a comprehensive security lifecycle. Effective remediation isn’t just about applying patches - it’s about creating a system of continuous oversight that ensures you're always prepared for regulatory checks. This proactive approach not only helps with compliance but also simplifies future security audits.
Best Practices for Reporting and Documentation
Keep all IoT inventory, risk assessments, threat analyses, and remediation actions in a centralized system. Doing so makes it easier to produce audit-ready reports. Using advanced analytics can further simplify this process by consolidating data efficiently, cutting down on manual work, and improving overall risk management [2][3].
Conclusion
Addressing healthcare IoT post-audit remediation requires a structured and thoughtful approach. This includes identifying vulnerabilities, creating a prioritized action plan, and implementing corrective measures that are thoroughly validated. Success hinges on keeping meticulous records and establishing systems for continuous monitoring to prevent future security lapses.
Prioritization plays a key role in tackling the most pressing risks first. Healthcare organizations should focus on vulnerabilities that endanger patient safety and data security, especially those involving life-critical devices such as ventilators, infusion pumps, and cardiac monitors. Collaborating closely with IoT device manufacturers ensures that necessary patches and updates are applied without compromising device functionality or violating regulatory standards. This method fits seamlessly into the broader risk management framework discussed earlier.
Given the challenge of managing potentially thousands of connected medical devices, centralized platforms are indispensable. Tools like Censinet RiskOps™ streamline remediation efforts by tracking progress and automating workflows, reducing manual tasks while still allowing for human oversight. Additionally, Censinet AITM speeds up the process by enabling quicker risk assessments and generating detailed compliance reports automatically.
Ongoing monitoring is crucial for tracking remediation progress, identifying new threats, and maintaining audit-ready documentation. By adopting this proactive stance, healthcare organizations can turn post-audit remediation into a continuous security effort - safeguarding patient care and staying ahead of regulatory demands.
FAQs
What are the key steps for effective post-audit remediation of IoT devices in healthcare?
To effectively address post-audit issues with healthcare IoT devices, the first step is creating a comprehensive inventory. This helps you catalog all devices and pinpoint their specific vulnerabilities. Once you have a clear picture, rank the risks based on their severity and how they might affect patient safety or compromise sensitive data.
From there, tackle these risks with focused solutions. This might include updating firmware, enforcing strong password policies, or applying critical security patches. These targeted actions are essential for minimizing risks.
Once the remediation steps are complete, make sure to monitor and validate the fixes regularly. This ensures that vulnerabilities are fully resolved and that the devices continue to meet compliance standards. By following this process, you can safeguard patient information, uphold the reliability of devices, and strengthen cybersecurity across healthcare systems.
How does Censinet RiskOps™ improve IoT remediation for healthcare organizations?
Censinet RiskOps™ makes managing IoT risks in healthcare faster and easier by simplifying risk assessments and automating essential workflows. This helps healthcare organizations respond to vulnerabilities more quickly and effectively.
The platform delivers practical insights specifically designed for healthcare IoT settings, enabling organizations to focus on the most pressing risks and apply targeted security measures. With improved speed and precision, Censinet RiskOps™ helps safeguard patient data, medical devices, and vital systems.
Why is it essential to continuously monitor IoT devices in healthcare after fixing vulnerabilities, and how can it be done effectively?
Keeping healthcare IoT devices secure and functional doesn’t stop at fixing vulnerabilities - it requires continuous monitoring. This ongoing vigilance is essential for spotting new threats and weaknesses early, safeguarding patient safety, and ensuring devices operate without disruption.
To make this process manageable, organizations can rely on automated tools that deliver real-time updates on device activity and security. Pairing these tools with regular audits and working closely with reliable vendors adds another layer of protection. This proactive mindset not only minimizes the risk of cyberattacks but also helps ensure the long-term dependability of healthcare IoT systems.
Related Blog Posts
Key Points:
What is post-audit remediation for IoT?
Post-audit remediation for IoT refers to the process of addressing vulnerabilities, compliance gaps, and security risks identified during an audit. This process ensures that IoT systems meet regulatory standards and are fortified against potential threats.
Why is post-audit remediation important for IoT?
Post-audit remediation is critical for maintaining the integrity and security of IoT systems. It helps organizations mitigate risks, comply with industry regulations, and protect sensitive data from breaches or unauthorized access.
What are the key steps in post-audit remediation?
The process typically involves:
- Identifying vulnerabilities: Reviewing audit findings to pinpoint weaknesses.
- Prioritizing risks: Assessing the severity of vulnerabilities to address critical issues first.
- Implementing fixes: Applying patches, updates, or configuration changes to resolve issues.
- Monitoring compliance: Continuously tracking system performance to ensure ongoing adherence to standards.
How does IoT security benefit from post-audit remediation?
Effective remediation enhances IoT security by reducing the likelihood of cyberattacks, ensuring compliance with regulations, and improving the overall resilience of connected devices and systems.
What tools can assist in IoT post-audit remediation?
Organizations can leverage tools such as:
- Vulnerability scanners: To identify and assess risks.
- Compliance management platforms: To track and document remediation efforts.
- Automated monitoring systems: To ensure continuous security and compliance.
What challenges are common in IoT post-audit remediation?
Common challenges include:
- Resource constraints: Limited budgets or personnel to address issues.
- Complex IoT ecosystems: Managing diverse devices and platforms.
- Evolving regulations: Keeping up with changing compliance requirements.


