HIPAA Compliance: Storing Audit Evidence Safely
Post Summary
When it comes to HIPAA compliance, securely storing audit evidence is non-negotiable. Audit evidence - like logs, policies, and training records - proves your organization protects electronic protected health information (ePHI) and adheres to federal regulations. Without proper storage, you risk fines, legal trouble, and operational setbacks.
Here’s what you need to know:
- Retention Rule: HIPAA requires you to keep key documentation for at least six years. Some state laws or contracts may demand longer periods.
- Audit Evidence Examples: Logs, risk analyses, access records, backup tests, and breach notifications.
- Storage Essentials: Use encryption, role-based access, and secure repositories (e.g., SIEM systems or HIPAA-compliant cloud storage).
- Tamper Prevention: Implement immutable storage (WORM) and cryptographic methods to ensure data integrity.
- Physical Security: Restrict access to storage areas with locks, monitoring systems, and strict visitor protocols.
- Destruction Protocols: When retention periods end, destroy records securely (e.g., shredding, degaussing) and document the process.
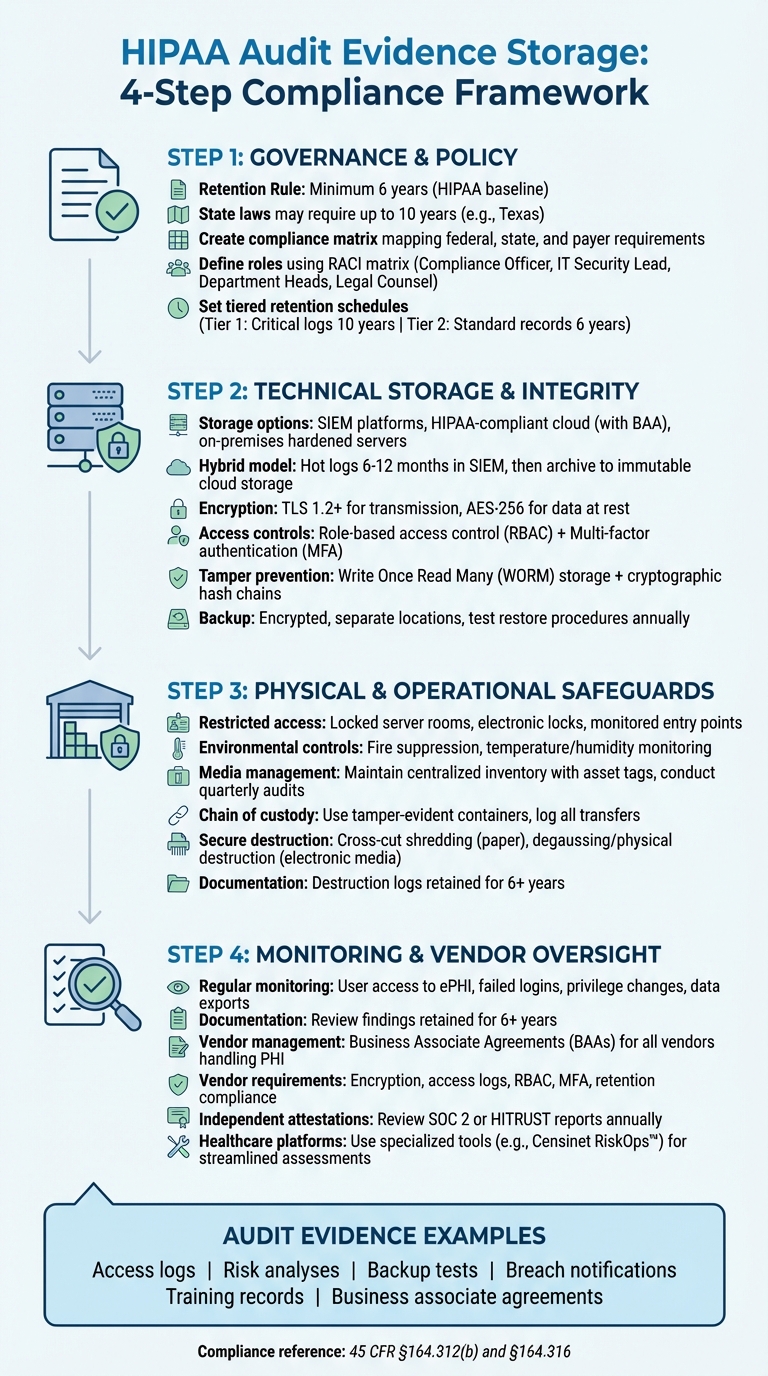
HIPAA Audit Evidence Storage: 4-Step Compliance Framework
Governance, Policy, and Retention Checklist
Identify Your Regulatory Requirements
Start by examining HIPAA's Security Rule (45 CFR §164.312(b)), which mandates the use of audit controls - tools like hardware, software, and procedures designed to track and analyze activity in systems containing electronic PHI. Additionally, the documentation requirement under 45 CFR §164.316 specifies that policies, procedures, and related records must be kept for at least six years from their creation date or the last time they were in effect [2][5].
While HIPAA establishes a baseline, state laws often require longer retention periods. For instance, Texas mandates up to 10 years for adult records, while Minnesota has extended requirements for records involving minors [2][6]. When state laws, payer contracts, or accreditation standards exceed HIPAA's requirements, the longer retention period takes precedence. To navigate these overlapping rules, conduct a jurisdictional review and create a compliance matrix. This matrix should clearly map federal, state, payer, and accreditation requirements to ensure you apply the correct retention period for each record type [2][3][6].
Define Audit Evidence Scope and Ownership
Once you've mapped out the regulatory requirements, inventory all systems that generate audit evidence and assign clear ownership for each. These systems might include electronic health records (EHRs) that track PHI access, billing software that logs user authentication, email servers that record PHI transmissions, and cloud storage platforms that log access events [3][9]. For each system, document the types of logs it generates (e.g., user logins, PHI views, data modifications) and estimate their volumes. Focus on high-risk systems like EHRs, and use NIST guidelines tailored to HIPAA requirements to prioritize your efforts.
Define roles and responsibilities using a RACI matrix (Responsible, Accountable, Consulted, Informed). For example:
- The Compliance Officer typically oversees governance and audit processes.
- The IT Security Lead handles technical aspects like system integrity and access controls.
- Department Heads provide operational context, such as verifying clinical logs.
- Legal Counsel ensures retention practices comply with applicable laws.
Include these assignments in your policies and specify any necessary training to ensure all stakeholders understand their roles and responsibilities.
Set Retention Schedules and Policies
After identifying system ownership, formalize retention schedules to maintain compliance across all records. Start with HIPAA's six-year minimum retention period and extend it as required by state laws, organizational policies, or contract obligations [2][3]. A tiered approach can simplify this process. For instance:
- Tier 1: Critical logs (e.g., EHR access logs) with a 10-year retention period.
- Tier 2: Standard records retained for six years.
For example, a healthcare provider in New York might specify in its master schedule that business associate agreements must be kept for six years after termination, with clear protocols for secure destruction.
Keep in mind that HIPAA's six-year retention period starts from the date a policy was last in effect - not when it was originally created. If a policy is revised after three years, the retention clock resets, requiring the older version to be retained for six more years [5]. Document the reasoning behind your retention timelines, implement automated lifecycle management tools (e.g., archiving records after one year and securely deleting them after year seven), and align these practices with your legal hold processes [3][5].
Technical Storage and Data Integrity Checklist
Set Up Secure Storage Repositories
When storing audit evidence, ensure your repositories comply with HIPAA 45 CFR §164.312 safeguards. Depending on your setup, you can use on-premises hardened servers if your data center is robust, Security Information and Event Management (SIEM) platforms to centralize logs from EHRs, clinical applications, firewalls, and identity systems, or HIPAA-compliant cloud storage with a signed Business Associate Agreement (BAA) [2][8][9]. Cloud storage provides scalable options with encryption, access logging, and regional redundancy [2][8].
A common approach among U.S. healthcare organizations is a hybrid model. They keep "hot" logs in a SIEM for 6–12 months for quick access during investigations, then archive older data in low-cost, immutable cloud storage for the rest of the retention period [10][3]. To secure storage further, disable unused services, close unnecessary ports, and adhere to NIST or CIS baselines [8]. Regularly patch operating systems and storage services as part of a documented vulnerability management program. Limit network access to trusted application servers and authorized administrators [2][8].
Apply Encryption and Access Controls
Once your storage repositories are secure, focus on encrypting and controlling access to the stored data. Although HIPAA considers encryption an "addressable" safeguard, it is widely treated as essential for protecting audit evidence [8]. Use TLS 1.2 or higher for transmitting logs, disable weak ciphers, and enable mutual authentication [2][8]. For data at rest, implement AES-256 encryption, manage encryption keys in a hardened key management system (KMS), and enable disk-level or volume encryption. Be sure to document encryption settings and key rotation policies [2][8].
Access to audit evidence should follow role-based access control (RBAC) principles, ensuring users only have the access necessary for their roles [2][8]. For example, security analysts might need access to search logs but not to modify storage policies, while compliance officers may review reports but not change system configurations. Protect privileged access with multi-factor authentication (MFA) using hardware tokens, authenticator apps, or enterprise SSO [2][8]. Sync access policies with central identity systems to reflect staffing changes promptly, and conduct quarterly access reviews to ensure only authorized personnel retain access [2]. Additionally, every interaction with the logs - whether viewing, exporting, or attempting modifications - should generate a meta-log entry to track activity [3][4].
Prevent Tampering and Maintain Backups
To protect audit evidence from tampering, use Write Once, Read Many (WORM) or immutable storage, ensuring log records cannot be altered or deleted until the retention period expires [2][4]. This can be achieved by locking objects or volumes for a set duration. Consider cryptographically chaining log entries - where each entry references the hash of the previous one - or hashing logs in batches and storing hash values separately. Any tampering would disrupt the hash chain [4]. Ensure administrative controls for modifying retention settings or WORM policies are tightly restricted and logged [2][4].
Backup and disaster recovery plans for audit evidence should align with HIPAA's contingency plan standards [8]. Encrypt backups, store them in separate locations - such as another data center or cloud region - and secure them with equal or stricter access controls than the primary repository [2][8]. Many organizations use a combination of daily full backups and frequent incremental backups, aligning retention policies with the primary system [2][3]. Regularly test your restore procedures - at least once a year - to confirm you can recover historical logs within your recovery time objectives (RTOs) and recovery point objectives (RPOs) [2][8]. These steps ensure your audit evidence remains secure, intact, and accessible when needed.
Physical and Operational Safeguards Checklist
Secure Physical Storage Locations
While encryption and access controls safeguard your digital data, physical security is just as essential when it comes to protecting audit evidence. HIPAA mandates that audit evidence be stored in restricted areas - like locked server rooms or records rooms - where public access is strictly prohibited [8]. These spaces should have strong physical barriers, electronic locks with tightly controlled key distribution, monitored entry points, and environmental controls such as fire suppression systems and temperature and humidity monitoring. These measures complement the technical safeguards already in place.
Extend your Role-Based Access Control (RBAC) policies to physical access. Only authorized personnel - such as IT security staff, compliance officers, or internal auditors - should have access to these storage areas via badges or keys [2]. Visitor protocols should include sign-ins, ID verification, badges, and escorts [2][5]. Regularly review physical access logs for any unusual activity [2]. Configure access control systems to trigger alerts for forced entries or access attempts outside of approved hours [8]. Keep all documentation of these reviews - like checklists, tickets, and remediation notes - for at least six years as required by HIPAA [2][5].
Manage Media and Devices
Securing storage areas is just one piece of the puzzle; the devices and media containing audit evidence also require protection. Maintain a centralized, up-to-date inventory that tracks asset tags, serial numbers, ownership, location, and status for every device or piece of media holding audit data [2][5]. Classify each item based on its data sensitivity and establish written procedures for how these items are issued, used, stored, and returned [2]. Use check-in logs and conduct quarterly audits to verify that all media remain accounted for [5].
When audit evidence needs to be transported - whether to data centers, offsite storage, or legal counsel - use tamper-evident containers with unique identifiers [5]. Record every transfer in a chain-of-custody log, detailing the date, time, sender, receiver, purpose, and condition of the evidence at the time of handoff [5]. Use trusted couriers with signed agreements for transportation, and train staff to keep evidence under their direct control at all times. Evidence should never be left unattended in vehicles or public spaces, and any signs of tampering must be reported immediately [8]. Chain-of-custody logs should integrate seamlessly with your inventory system so you always know the location and custodian of each item. Like other records, these logs must be kept for at least six years when they document security measures [2][5].
Destroy Evidence Securely and Document It
Once physical and media safeguards are in place, the next step is implementing secure destruction protocols. Protected Health Information (PHI) must be destroyed in a way that renders it unreadable [5][8]. For paper records, acceptable methods include cross-cut shredding, pulping, or incineration. For electronic media, options include degaussing, physical destruction (like shredding or crushing), or secure wiping using industry-recognized standards [5]. Keep in mind that some media, like USB drives, have limited data retention reliability and may need to be replaced and destroyed earlier than expected [5].
Every destruction event should be meticulously logged. A destruction log should include details such as the date, type and volume of records or media destroyed, unique identifiers or batch IDs, destruction method, location, and the names and signatures of the individuals or vendors who performed and witnessed the destruction [5]. If you work with third-party destruction vendors, make sure to obtain certificates of destruction that provide similar details. Retain these certificates for at least six years as part of HIPAA's documentation requirements [2][5]. These records should link back to your master inventory, allowing you to demonstrate the full lifecycle of each evidence item - from creation and storage to its final destruction. This thorough documentation aligns with HIPAA's retention and compliance standards, ensuring you're prepared for any regulatory or legal scrutiny [2][7].
sbb-itb-535baee
Monitoring, Validation, and Vendor Oversight Checklist
Schedule Regular Monitoring and Reviews
Under HIPAA's Security Rule, healthcare organizations are required to regularly review audit logs, access reports, and security incident tracking [8]. To meet this requirement, many organizations use automated monitoring systems that provide near real-time oversight, supplemented by regular reviews of exceptions [3]. Key areas to monitor include user access to electronic protected health information (ePHI), failed login attempts, privilege changes, configuration updates, and data exports.
Specific roles, such as a Security or Privacy Officer, should be assigned to review anomalies and escalate them as needed [2][9]. Each review should be documented with details like the date, systems reviewed, findings, and any remediation actions taken [2][3]. This documentation must be retained for at least six years to comply with HIPAA's record-keeping requirements [2][5]. Additionally, access control systems should be configured to send alerts for unauthorized access attempts or activity outside approved hours [8].
It’s also important to extend these monitoring practices to vendors and cloud providers to ensure compliance across third-party services.
Manage Vendors and Cloud Providers
Establish Business Associate Agreements (BAAs) with all vendors handling PHI, ensuring they adhere to HIPAA safeguards and secure-destruction protocols. A vendor oversight program should include standardized questionnaires to confirm that providers encrypt audit data during transmission and storage, maintain detailed access logs, enforce role-based access and multi-factor authentication, and comply with HIPAA’s retention and secure destruction guidelines [2][3][5][8].
Independent attestations like SOC 2 or HITRUST reports should be reviewed, with a focus on logging, data retention, and incident response measures. Set regular recertification cycles - typically annual for high-risk vendors - and schedule off-cycle reviews when incidents or significant service changes occur [2][5].
For a streamlined approach, consider using platforms tailored to the healthcare industry.
Use Healthcare-Specific Risk Management Platforms
Platforms like Censinet RiskOps™ are designed to simplify third-party risk assessments in healthcare. They centralize vendor intake and reassessment workflows, maintain a complete, time-stamped record of due diligence, and document remediation activities - all of which strengthen audit evidence for HIPAA compliance. These platforms facilitate the distribution and evaluation of vendor security questionnaires, focusing on encryption, access controls, log retention, and secure destruction practices. They also benchmark vendors against others in the healthcare sector to spot emerging risks. By integrating with ticketing or governance, risk, and compliance (GRC) systems, these platforms ensure vendor-related findings are tracked and resolved, supporting both OCR and internal audits.
"Healthcare is the most complex industry... You can't just take a tool and apply it to healthcare if it wasn't built specifically for healthcare." - Matt Christensen, Sr. Director GRC, Intermountain Health [1]
For example, integrating Censinet RiskOps™ with your Security Information and Event Management (SIEM) system can automate vendor reassessments when significant changes occur, such as shifts in infrastructure regions or updates to encryption protocols. The resulting evidence can then be attached to your centralized HIPAA documentation repository [2][5].
Conclusion
Storing audit evidence securely is essential for maintaining patient trust, ensuring seamless clinical operations, and protecting your organization from hefty regulatory penalties. When it comes to HIPAA enforcement, the ability to demonstrate what actions were taken, when they occurred, and under which controls is often critical [2][7]. Missing, incomplete, or altered audit evidence can weaken your defense during an OCR investigation, increasing the likelihood of fines and corrective action plans [7].
A strong audit evidence program weaves together governance, technical safeguards, physical security, and ongoing monitoring. Start by creating policies that clearly define audit evidence, assign accountability, and specify retention periods [2][5]. Use advanced technical safeguards to ensure the integrity of your data [2][8]. Protect physical storage areas, handle media with care, and document proper destruction when retention periods expire [2][5].
Regularly validating and monitoring your controls ensures they work as intended over time. This includes reviewing logs, testing backups, and updating risk assessments periodically [2][5][7]. For third-party vendors and cloud providers, it’s crucial to have Business Associate Agreements in place, evaluate their controls, and confirm they meet log retention and encryption requirements [2][5][7]. These practices lay the foundation for leveraging specialized tools to further enhance compliance.
Healthcare organizations can benefit from platforms like Censinet RiskOps™, which streamline vendor assessments, track remediation efforts, and provide a clear record of oversight for HIPAA audit readiness [2][3]. By combining governance, technology, and continuous oversight, you can shift from reactive compliance efforts to a proactive, sustainable approach that strengthens your overall business operations [2][3].
FAQs
What are the key steps to securely encrypt audit evidence for HIPAA compliance?
To ensure the secure encryption of audit evidence and meet HIPAA requirements, consider these essential practices:
- Use robust encryption algorithms: Opt for methods like AES-256 to protect sensitive information effectively.
- Encrypt data at all stages: Safeguard data both when stored and during transmission to prevent unauthorized access.
- Enforce strict access controls: Limit decryption capabilities to authorized personnel only.
- Regularly update encryption keys: Use secure key management protocols to minimize potential vulnerabilities.
- Keep detailed audit logs: Monitor access and decryption activities to maintain accountability and transparency.
By implementing these measures, healthcare organizations can better protect audit evidence while staying compliant with HIPAA standards.
How can healthcare organizations determine the appropriate retention period for audit evidence under state laws?
Healthcare organizations need to figure out how long to keep audit evidence by checking their state's specific regulations. These rules often differ and may specify varying retention periods depending on the type of records or data.
To stay compliant, it’s a good idea to work with legal counsel or compliance professionals who understand both state laws and HIPAA guidelines. By aligning retention policies with these regulations and industry practices, organizations can protect audit evidence while adhering to privacy and security requirements.
How should healthcare organizations securely dispose of audit evidence after the retention period ends?
To safely dispose of audit evidence once the retention period has passed, healthcare organizations must first verify that the data is no longer required for legal, regulatory, or operational reasons. Always adhere to established policies that align with HIPAA guidelines to ensure secure handling. Employ reliable destruction methods like shredding, degaussing, or certified data-wiping tools to ensure the information is completely erased.
Make sure to document the destruction process thoroughly. Record details such as the date of destruction, the method used, and the personnel involved to maintain a clear and traceable audit trail. Additionally, regularly review and update your data destruction policies to remain compliant with changing regulations and industry standards.


