Medical Device Vendor Risk Management: FDA Compliance and Patient Safety Best Practices
Post Summary
Medical device vendor risk management involves assessing and mitigating risks associated with third-party vendors to ensure compliance, patient safety, and device reliability.
FDA compliance ensures that medical devices meet safety, efficacy, and quality standards, reducing risks to patients and avoiding regulatory penalties.
Risks include cybersecurity vulnerabilities, non-compliance with FDA or ISO standards, supply chain disruptions, and poor-quality components.
Vendor failures can lead to device malfunctions, inaccurate results, delayed treatments, and increased risks of harm to patients.
Best practices include conducting thorough vendor assessments, adhering to ISO 14971 standards, implementing quality control measures, and maintaining robust documentation.
Risk management reduces device failures, ensures compliance, and enhances reliability, ultimately protecting patients and improving outcomes.
Medical device vendor risk management ensures compliance with FDA regulations while protecting patient safety. It involves assessing, monitoring, and mitigating risks tied to third-party vendors. Poor oversight can lead to regulatory penalties, device failures, or data breaches. Key strategies include:
- Following FDA regulations like 21 CFR Part 820 and 21 CFR Part 11 for quality systems and electronic records.
- Using risk assessment methods such as FMEA, FTA, and Threat Modeling to identify and address potential issues.
- Conducting evaluations throughout the device lifecycle: pre-market, implementation, and post-market phases.
- Leveraging tools like Censinet RiskOps™ for automated assessments, compliance tracking, and real-time monitoring.
Effective vendor risk management safeguards patient safety, ensures regulatory compliance, and minimizes financial and reputational risks. Focus on prioritizing high-risk vendors, maintaining thorough documentation, and using advanced tools to streamline processes.
FDA Regulations for Medical Device Vendor Risk
The FDA enforces stringent guidelines to ensure that medical device vendors comply with regulations aimed at protecting patient safety.
Current FDA Requirements and Standards
The backbone of medical device quality management in the U.S. is 21 CFR Part 820, a regulation that outlines the essential procedures manufacturers must follow. These include the design, production, packaging, labeling, storage, installation, and maintenance of medical devices. To meet these requirements, manufacturers are obligated to validate their suppliers, maintain thorough documentation, and regularly assess risks, implementing corrective actions when necessary.
In addition to domestic regulations, international standards like ISO 14971:2019 provide a framework for managing risks throughout a medical device's lifecycle. This standard encourages organizations to apply risk management principles not just internally but also in their dealings with third-party vendors.
Another key regulation is 21 CFR Part 11, which governs the use of electronic records and signatures. This rule ensures that vendor risk management activities are properly documented through secure audit trails, data integrity measures, and user authentication protocols.
Ignoring these requirements isn’t just a regulatory misstep - it can lead to serious consequences, as outlined below.
Risks of Non-Compliance
Failing to comply with FDA regulations for vendor risk management can trigger severe repercussions. Regulatory responses may include warning letters, consent decrees, or even bans on importing medical devices. In extreme cases, non-compliance could lead to criminal charges and expensive remediation efforts.
The risks extend beyond regulatory penalties. Poor vendor oversight can jeopardize patient safety by causing device malfunctions, exposing sensitive data, or disrupting supply chains - any of which can compromise care. Financially, non-compliance can result in higher insurance premiums, legal fees, and the costs of corrective actions. On top of that, reputational damage from these issues can erode public trust and make future collaborations more challenging.
Risk Assessment Methods and Tools
Structured risk assessments play a crucial role in identifying and addressing issues that could impact patient care. Below, we’ll explore some effective methods for evaluating and managing vendor risks while ensuring compliance and prioritizing patient safety.
Primary Risk Assessment Methods
Failure Mode and Effects Analysis (FMEA) is a widely used method in healthcare for identifying potential failures and their impacts. It works by pinpointing failure modes, assessing their effects, and determining root causes. For medical device vendors, this could include risks like supply chain disruptions, quality control issues, or cybersecurity vulnerabilities. Each risk is assigned a Risk Priority Number (RPN), calculated based on severity, likelihood of occurrence, and difficulty of detection. This scoring system helps prioritize mitigation efforts and supports compliance with FDA standards.
Fault Tree Analysis (FTA) takes a reverse-engineering approach. Starting with an undesired event, it traces back to uncover all possible causes. This method is especially useful for analyzing complex vendor-related incidents or preparing for potential failures. FTA visually maps out these relationships using tree diagrams, making it easier to understand how smaller issues could lead to significant problems.
Healthcare organizations often rely on FTA when assessing critical vendor relationships, such as those involving life-support systems or surgical devices. It helps identify single points of failure and reveals how seemingly minor issues could escalate into significant patient safety risks.
Threat Modeling is a focused method for assessing security risks, particularly for connected devices. It identifies potential attack vectors, evaluates existing security controls, and highlights areas needing improvement. In vendor risk management, threat modeling helps organizations understand how third-party relationships could expose them to cyberattacks or data breaches.
This method typically revolves around four key questions: What are we protecting? What are we protecting against? How well are we protecting it? Have we done a good job? Applying this framework to vendor relationships enables healthcare organizations to assess security vulnerabilities and implement effective safeguards.
Risk Assessment Throughout Device Lifecycle
Risk assessment is not a one-and-done process - it evolves throughout a medical device’s lifecycle to adapt to changing circumstances. Here’s how it unfolds across different phases:
- Pre-market phase: This stage involves conducting detailed vendor evaluations, focusing on financial stability, quality management systems, regulatory compliance history, and technical capabilities. These initial assessments establish baseline risks and determine whether to proceed with a vendor.
- Implementation phase: During this period, continuous monitoring is essential as vendors integrate their devices into healthcare settings. Risk assessments should address installation quality, staff training, and early performance indicators. The goal is to catch any emerging issues that may not have been evident during earlier evaluations.
- Post-market surveillance: This is the longest and most critical phase. Healthcare organizations must track device performance, monitor adverse events, assess vendor responsiveness, and ensure ongoing regulatory compliance. This phase often uncovers risks like long-term reliability issues or new cybersecurity threats. Regular reassessments, including annual comprehensive reviews and more frequent checks for high-risk vendors, are vital for effective management.
Risk Assessment Tool Comparison
Each risk assessment method offers unique strengths and limitations, making them suitable for different scenarios. The table below compares their applications:
| Method | Best Use Cases | Key Advantages | Primary Limitations |
|---|---|---|---|
| FMEA | Process evaluation, quality management | Quantitative scoring, systematic identification | Time-intensive, requires detailed process knowledge |
| FTA | Incident investigation, critical system analysis | Visual representation, logical structure | Focuses on single events, less effective for ongoing monitoring |
| Threat Modeling | Cybersecurity and data protection | Security-centric, targets modern threats | Limited to security risks, requires specialized expertise |
The choice of method often depends on the type of risks being assessed and the resources available. Many healthcare providers combine these approaches for a more thorough evaluation. For instance, FMEA might be used for initial vendor assessments, threat modeling for cybersecurity evaluations, and FTA for investigating specific failures or incidents.
Hybrid approaches are also gaining traction. These combine elements from multiple methods, such as integrating FTA’s logical analysis into FMEA’s structured process or adding threat modeling’s security focus to ongoing evaluations. The goal is to align the chosen methods with the organization’s risk management priorities while ensuring comprehensive coverage of potential vendor risks.
Medical Device Vendor Risk Management Best Practices
Building solid vendor relationships right from the start is key to ensuring smooth collaboration across clinical, IT, compliance, and other teams. This approach helps everyone stay on the same page when it comes to meeting regulatory requirements and prioritizing patient safety. Early involvement allows teams to exchange insights, spot potential risks, and align their risk management goals effectively. With this groundwork in place, it becomes easier to conduct thorough vendor audits, maintain ongoing monitoring, and implement security measures with transparency.
sbb-itb-535baee
How Censinet Supports Medical Device Vendor Risk Management
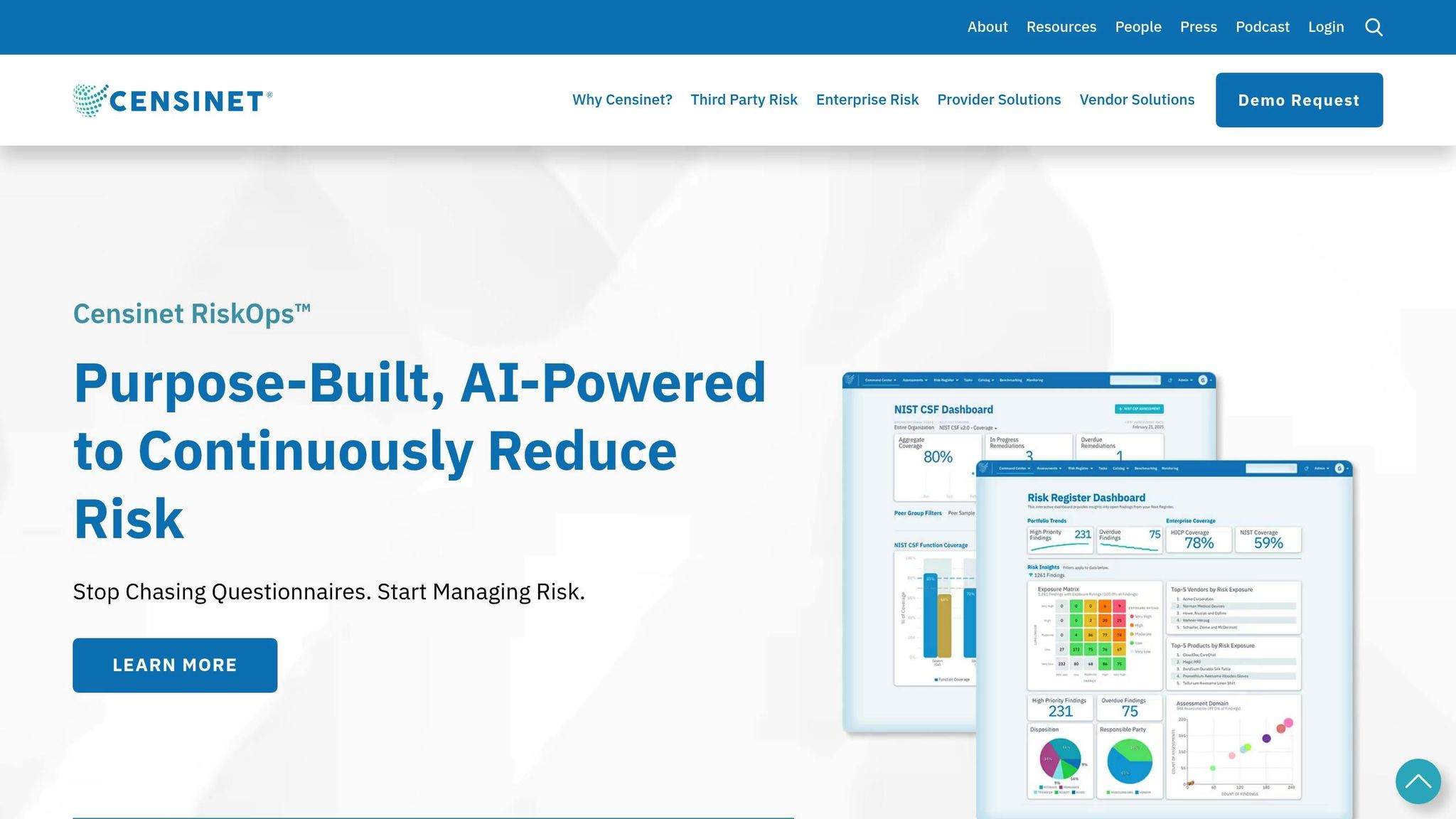
Managing vendor risks in healthcare requires precision, efficiency, and adherence to strict FDA compliance standards. Censinet streamlines this process with an automated, AI-powered platform designed to simplify risk assessments and compliance tracking. By replacing manual workflows, the platform improves both accuracy and speed, making vendor risk management more efficient.
Automated Risk Assessments with Censinet RiskOps™
Censinet RiskOps™ takes the hassle out of vendor risk assessments by automating the entire process. From sending and collecting risk questionnaires to analyzing the results, the platform minimizes manual data entry and reduces the chances of human error.
This automation significantly improves operational efficiency. Organizations can monitor risk status in real time, speeding up decision-making and enabling quicker responses to potential issues. The platform also flags critical concerns automatically, ensuring nothing slips through the cracks.
For healthcare organizations juggling multiple medical device vendors, real-time risk tracking is a game-changer. The system provides a clear, comprehensive view of all vendor relationships, capturing every assessment and ensuring compliance with FDA requirements for ongoing risk management. Its electronic questionnaire system standardizes data collection, reducing the risk of missing vital information during inspections and creating a solid foundation for informed, risk-based decisions.
AI-Powered Efficiency and Scale
Censinet AI™ enhances the platform's capabilities by automating routine tasks like evidence review and documentation analysis. The AI tools efficiently process large volumes of data, flag outdated or missing information, recommend corrective actions, and generate detailed risk reports. These reports combine assessment data, vendor evidence, and compliance requirements into a cohesive view of vendor risk.
The AI also identifies inconsistencies automatically, allowing risk teams to focus their expertise on high-priority issues rather than routine data checks. This approach enables organizations to expand their risk management programs without needing additional staff or resources.
To ensure accountability, the platform incorporates checkpoints where risk managers review AI-generated findings, validate scores, and make final decisions. Complex or ambiguous cases still rely on human expertise, ensuring that automation supports - rather than replaces - critical decision-making.
The platform’s evidence validation tools keep vendor information accurate and up to date. It monitors changes in vendor status, regulatory requirements, or risk profiles, automatically triggering reassessments when necessary. This ensures that risk evaluations remain current throughout the lifecycle of medical devices.
Supporting Compliance and Patient Safety
Censinet’s platform goes beyond automation and AI to reinforce compliance and patient safety. It aligns with FDA regulatory requirements by documenting risk assessments, tracking corrective actions, and maintaining detailed audit trails. It supports QMSR compliance by categorizing vendors, monitoring performance, and organizing documentation for easy access during inspections.
The platform also addresses cybersecurity compliance in line with recent FDA guidance. Integrated tools manage software bills of materials (SBOMs), monitor vulnerabilities, and document security controls, ensuring healthcare organizations meet the growing expectations for cybersecurity documentation during FDA inspections.
With its comprehensive record-keeping, Censinet maintains detailed logs of risk assessments, vendor communications, corrective actions, and approvals. These records are exportable and formatted for FDA reviews, helping organizations demonstrate compliance with regulatory standards for risk management and supplier oversight.
Proactive compliance monitoring is another key feature. The system schedules periodic reassessments, sends vendors reminders for updated documentation, and tracks changes in regulatory requirements. This ensures that risk profiles stay current and emerging risks are addressed promptly, meeting FDA expectations for post-market surveillance.
Finally, dashboard analytics and reporting provide healthcare organizations with critical insights, such as vendor risk scores, assessment progress, remediation timelines, and overall compliance status. These metrics help identify trends, benchmark performance, and focus resources on high-risk vendors, driving continuous improvement in risk management strategies.
Key Points for Medical Device Vendor Risk Management
Effective vendor risk management in the medical device industry requires balancing regulatory compliance, as outlined by FDA standards, with operational efficiency. Healthcare organizations that excel in this area build stronger safeguards against potential risks.
Best Practices Overview
A systematic risk framework is the backbone of any successful vendor management program. Combining ISO 14971 guidelines with project management principles creates a comprehensive approach that includes Risk Planning, Risk Assessment, Risk Monitoring and Control, and Risk Reporting.
Prioritizing risks ensures resources are directed where they matter most. By analyzing risks based on their severity and likelihood, organizations can focus on high- and medium-priority threats. This ensures that critical risks, such as those posing life-threatening dangers or risks of serious injury, are addressed immediately, while less pressing issues are monitored routinely.
Thorough documentation and audit trails are crucial for regulatory compliance. Detailed records of risk assessments, vendor communications, corrective actions, and approval decisions not only demonstrate adherence to FDA requirements but also support ongoing risk management throughout the device's lifecycle.
Collaboration among teams - including clinical, IT, procurement, and compliance departments - strengthens risk management efforts. Clear roles and responsibilities help organizations identify and address risks more efficiently, ensuring swift responses to emerging issues.
These practices lay the groundwork for improving vendor risk management processes.
Steps to Take
Begin by assessing your current vendor risk management practices against FDA standards and industry guidelines. Develop a detailed risk planning phase that defines your strategy, methodology, roles, and criteria for evaluating risk probability and impact.
Consider adopting automated risk assessment tools to improve efficiency and reduce manual errors. These platforms simplify tasks like distributing questionnaires, collecting data, and analyzing results, while still allowing human oversight for complex decisions.
Focus on high-priority vendor relationships first - especially those involving Class II and Class III medical devices or vendors with access to patient data and clinical systems. Establish regular reassessment schedules and create clear escalation procedures for addressing high-priority risks.
For organizations looking to modernize their approach, explore technology platforms that integrate risk assessment, compliance tracking, and monitoring into one streamlined workflow. The right technology partner can turn vendor risk management into a strategic tool that enhances patient safety and operational effectiveness.
FAQs
How does a structured risk framework improve vendor risk management for medical devices?
A structured risk framework streamlines vendor risk management by providing a well-defined process to identify, evaluate, and mitigate potential risks associated with third-party medical device vendors. This approach allows organizations to proactively spot vulnerabilities, gauge their potential impact, and implement specific measures to minimize those risks.
By adhering to regulatory standards such as FDA guidelines and ISO 14971, these frameworks not only ensure compliance but also emphasize patient safety. They facilitate continuous monitoring and reporting, enabling organizations to adapt to changing risks while maintaining exceptional standards of care.
What are the risks of not complying with FDA regulations for medical device vendors?
Failing to meet FDA regulations can lead to serious consequences for medical device companies. These consequences might include steep fines, product recalls, or even losing FDA approval altogether. For instance, civil monetary penalties can climb as high as $100,000 for specific violations, and businesses may face injunctions that could completely halt their operations.
Beyond the financial and legal fallout, non-compliance can severely harm a company's reputation. This loss of trust can make it harder to compete in the market and may hinder future growth opportunities. But the impact doesn't stop there - non-compliance can disrupt patient care, creating risks to public health and safety. Following FDA regulations isn’t just about avoiding penalties; it’s about safeguarding both patients and the future of your business.
How does Censinet RiskOps™ streamline vendor risk assessments for medical device compliance and patient safety?
Censinet RiskOps™ simplifies how vendor risk assessments are handled by automating essential tasks such as data collection, evaluation, and risk scoring. This approach speeds up the process, improves accuracy, and minimizes the likelihood of human mistakes.
With its standardized workflows and real-time insights, healthcare organizations can pinpoint and tackle potential risks efficiently. By adhering to FDA regulations and keeping patient safety at the forefront, Censinet RiskOps™ establishes a dependable system for managing third-party medical device vendors.
Related Blog Posts
Key Points:
What is medical device vendor risk management, and why is it important?
- Definition: Medical device vendor risk management is the process of identifying, assessing, and mitigating risks associated with third-party vendors to ensure device safety, reliability, and compliance.
- Importance: Vendors provide critical components, software, and services for medical devices. Poor vendor performance can lead to device failures, regulatory violations, and patient harm.
- Focus Areas: Risk management addresses vendor reliability, cybersecurity, regulatory compliance, and quality assurance.
Why is FDA compliance critical for medical devices?
- Safety Standards: FDA compliance ensures that medical devices meet stringent safety and efficacy requirements.
- Regulatory Adherence: Non-compliance can result in fines, recalls, and loss of market access.
- Patient Protection: FDA regulations, such as those outlined in ISO 14971, prioritize reducing risks to patients and ensuring device reliability.
- Market Access: Compliance with FDA and international standards is essential for entering global markets.
What are common risks in medical device vendor management?
- Cybersecurity Vulnerabilities: Devices connected to networks are at risk of hacking, data breaches, and ransomware attacks.
- Non-Compliance: Vendors failing to meet FDA or ISO 14971 standards can jeopardize device approval and market access.
- Supply Chain Disruptions: Delays or shortages in critical components can halt production and impact patient care.
- Poor-Quality Components: Substandard materials or manufacturing processes can lead to device malfunctions and patient harm.
How do vendor failures impact patient safety?
- Device Malfunctions: Faulty components or software can cause devices to fail, leading to inaccurate diagnoses or treatments.
- Delayed Treatments: Supply chain issues or system failures can delay critical medical interventions.
- Increased Risks: Poor vendor oversight can result in devices that do not meet safety standards, increasing the likelihood of adverse events.
- Regulatory Violations: Non-compliance with FDA or ISO standards can lead to recalls, fines, and reputational damage.
What are best practices for managing medical device vendor risks?
- Thorough Vendor Assessments: Evaluate vendors for compliance with FDA, ISO 14971, and other relevant standards.
- Adherence to ISO 14971: Implement risk management processes that address the entire device lifecycle, from design to decommissioning.
- Quality Control Measures: Regularly audit vendor processes and products to ensure consistent quality.
- Robust Documentation: Maintain detailed records of risk assessments, vendor evaluations, and compliance activities to meet regulatory requirements.
- Cybersecurity Protocols: Ensure vendors implement strong cybersecurity measures to protect devices and patient data.
How does risk management improve patient safety?
- Error Reduction: Identifying and mitigating risks reduces the likelihood of device malfunctions and adverse events.
- Compliance Assurance: Adhering to FDA and ISO standards ensures devices meet safety and quality requirements.
- Enhanced Reliability: Proactive risk management improves device performance and reduces downtime.
- Patient Trust: Reliable and safe devices build confidence in healthcare services and improve patient outcomes.


